 Website:
Alcon
Website:
Alcon
Catalog excerpts

10-500-088-012 PRODUCT INFORMATION Alcon Laboratories, Inc. STERILE UV-Absorbing Acrylic Foldable Multipiece Posterior Chamber Lenses ENGLISH PRODUCT INFORMATION…………………………
Open the catalog to page 1
PRODUCT INFORMATION Alcon Laboratories, Inc. STERILE UV-Absorbing Acrylic Foldable Multipiece Posterior Chamber Lenses CAUTION: Federal (USA) law restricts this device to the sale by or on the order of a physician. Model Characteristics Chart Model Optic Type Haptic Angle DESCRIPTION AcrySof® UV-absorbing acrylic foldable multipiece posterior chamber lenses are optical implants for the replacement of the human crystalline lens in the visual correction of aphakia in adult patients following cataract surgery. The optical portion consists of a high refractive index soft acrylic material. This...
Open the catalog to page 2
Figure 2 SPECTRAL TRANSMITTANCE CURVES (PERCENTAGE OF ULTRAVIOLET TRANSMITTANCE) NOTES: • The cutoff wavelength and the spectral transmittance curves presented here represent the range of transmittance values of IOLs made from acrylate/methacrylate copolymer with bonded UV-absorber. • Measurements were direct transmittance using a 6 mm aperture and a disc of thickness equivalent to the optic center. • UV cutoff at 10% T is 395 nm (-5 diopter lens). UV cutoff at 10% T is 400 nm (+30 diopter lens). • Human lens data from Boettner and Wolter (1962). MODE OF ACTION AcrySof® posterior chamber...
Open the catalog to page 3
WARNINGS 1. As with any surgical procedure, there is risk involved. Potential complications accompanying cataract or implant surgery may include, but are not limited to the following: corneal endothelial damage, infection (endophthalmitis), retinal detachment, vitritis, cystoid macular edema, corneal edema, pupillary block, cyclitic membrane, iris prolapse, hypopyon, and transient or persistent glaucoma. 2. The safety and effectiveness of intraocular lens implants have not been substantiated in patients with preexisting ocular conditions (chronic drug miosis, glaucoma, amblyopia, diabetic...
Open the catalog to page 4
DIRECTIONS FOR USE 1. Examine the label on the unopened package for model, power, proper configuration, and expiration date. 2. After opening the cardboard storage container, verify lens case information (e.g., model, power, and serial number) is consistent with information on outer package labeling. 3. This device is sterile until the inner pouch is opened. Inspect the pouch carefully for tears, cuts, punctures or other signs that the pouch has been opened or damaged. DO NOT implant the IOL if the sterility has been compromised (see RETURNED GOODS POLICY). 4. To remove the lens, open the...
Open the catalog to page 5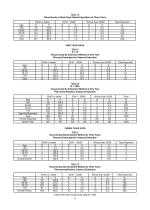
Table 1A Visual Acuity in Best Case Patient Population at Three Years Age <60 60-69 70-79 >79 Total ONE YEAR DATA Table 2 N = 18 Visual Acuity By Extraction Method at One Year Planned Extracapsular Cataract Extraction Age <60 60-69 70-79 >79 Total Table 2A N = 1020 Visual Acuity By Extraction Method at One Year Phacoemulsification Cataract Extraction Age <60 60-69 70-79 >79 Age Not Reported Total VA Not Reported Grand Total THREE YEAR DATA Table 3 Visual Acuity By Extraction Method at Three Years Planned Extracapsular Cataract Extraction Age <60 60-69 70-79 >79 Overall ECCE Table 3A Visual...
Open the catalog to page 6
COMPLICATIONS The United States Food and Drug Administration has identified eleven (11) potentially sight‑threatening complications which may occur following cataract extraction and/or intraocular lens implantation. The cumulative and persistent (present at the one year visit) rates of these complications during the first and third postoperative years for the Model MA60BM patients stratified by cataract extraction method is shown in Tables 4, 4A, 5 and 5A. Complication rates include all enrolled subjects for whom a case report form was received. Table 4 Cumulative Postoperative...
Open the catalog to page 7
Table 5A Persistent Postoperative Complications at Three Years ECCE N = 24 Corneal Edema Macular Edema Pupillary Block Secondary Glaucoma Cyclitic Membrane Vitritis Endophthalmitis Retinal Detachment Lens Dislocation Fifty-four (4.4%) patients receiving the AcrySof® Model MA60BM posterior chamber lens experienced one or more of the complications listed in the previous tables. However, the majority of these complications occurred early in the postoperative time frame and appeared to be associated with the cataract extraction. NOTE: Cumulative refers to any complication reported at any time...
Open the catalog to page 8
Table 6A Adverse Reactions at Three Years Postoperatively ECCE N = 24 Intraocular Infection Acute Corneal Decompensation Secondary Surgical Intervention: a) Lens Replacement/Removal b) Retinal Detachment Repair f) Wound Repair Leak g) Photocoagulation h) Removal of Residual Cortex Material SUPPLEMENTAL PUBLISHED DATA A prospective, well-controlled and randomized study (Ursell et al, 1998; Hollick et al. #1. 1998) with AcrySof® lenses utilizing a uniquely-developed coaxial illumination method linked to a novel computerized digital imaging system (Pande et al, 1998) was conducted with a total...
Open the catalog to page 9
Table 8 Posterior (Nd:YAG) Capsulotomy Rates IOL Material @ 3 years N % P value In addition, the reduced area of LECs observed in this study was associated with decreased lens epithelial cell proliferation (Hollick et al, #2. 1998) and anterior capsule movement (Ursell et al, 1997) for AcrySof® lenses as compared to the models of similarly designed silicone and PMMA lenses. HOW SUPPLIED These posterior chamber intraocular lenses are supplied dry, in a package terminally sterilized with ethylene oxide, and must be opened only under aseptic conditions (See DIRECTIONS FOR USE). EXPIRATION DATE...
Open the catalog to page 10
SYMBOLS USED ON LABELING SYMBOL Intraocular lens Posterior chamber Posterior chamber lens Anterior Asymmetric Biconvex Body diameter (Optic diameter) Overall diameter (Overall length) Do not reuse Use by Sterilized by ethylene oxide Serial number Attention: See instructions for use Manufacturer EC Authorized Representative in the European Community Upper Limit of Temperature Alcon Laboratories, Inc. 6201 South Freeway Fort Worth, Texas 76134-2099 USA Alcon Laboratories (UK) Ltd. Frimley Business Park Frimley, Camberley Surrey, GU16 7SR, United Kingdom © 2000, 2013-2014 Novartis
Open the catalog to page 11All Alcon catalogs and technical brochures
-
PUREPOINT
2 Pages
-
Vektor
2 Pages
-
NEGENUITY
2 Pages
-
CONSTELLATION®
4 Pages
-
Maintaining Good Eye Health
3 Pages
-
The LenSx® Laser System
12 Pages
-
CZ70BD
7 Pages
-
MODEL SN6AT3, SN6AT4, SN6AT5
23 Pages
-
MODEL SN60AT
9 Pages
-
MODEL SN6AD1
26 Pages
-
MODEL MA30AC
11 Pages
-
MODEL MN60AC
13 Pages
-
MODEL SN60WF
15 Pages
-
MODEL SN6CWS
15 Pages
-
NAPHCON A
1 Pages
-
PUREPOINT
6 Pages
-
CONSTELLATION® Vision System
10 Pages


























