Website:
medin Medical Innovations GmbH
Website:
medin Medical Innovations GmbH
Group: Hamilton Medical AG
Catalog excerpts

User manual for medinCNO® A Hamilton Medical Company
Open the catalog to page 1
The medinCNO® contains batteries and electrical components. Consequently it cannot be disposed of in domestic waste but must be collected separately and recycled in accordance with local regulations. (WEEE) 0483 Classification (according to COUNCIL DIRECTIVE 93/42/EEC Annex IX): IIb medin Medical Innovations GmbH Adam-Geisler-Str. 1 – 82140 Olching – Germany +49 8142-44846-0 – www.medin-medical.com – info@medin-medical.com Made in Germany medin Medical Innovations GmbH Adam-Geisler-Str. 1 D-82140 Olching
Open the catalog to page 2
medin Medical Innovations GmbH S +49 8142 448 460 OP_medinCNO EN Adam-Geisler-Str. 1 info@medin-medical.com Rev 12 Stand 06.03.2019
Open the catalog to page 3
A Hamilton Medical Company medin Medical Innovations GmbH S +49 8142 448 460 OP_medinCNO EN Adam-Geisler-Str. 1 info@medin-medical.com Rev 12 Stand 06.03.2019
Open the catalog to page 4
These instructions for use contain information about starting up, using and maintaining the medinCNO® (medin-cno). They also contain safety information, describe the device's functions and give an overview of the ancillary equipment needed. Users must be very familiar with the information and warnings given in these instructions for use in order to operate the medinCNO® safely. However, they are no substitute for training. The instructions and warnings are categorised as follows: Warning: Warnings must be followed in order to prevent possible serious consequences for the patient or the...
Open the catalog to page 5
A Hamilton Medical Company 2.1 Intended purpose The medinCNO® CPAP driver is used in combination with the Medijet® nCPAP generator to administer CPAP therapy to premature infants and newborns. The medinCNO® must be used under the supervision of expert, specially trained staff in a clinical setting, and the patient's oxygen saturation must be monitored at the same time. Warning: - The medinCNO® is intended for clinical use only. - The medinCNO® may only be used while the patient's oxygen saturation is being monitored at the same time. - The medinCNO® may only be used by and under the...
Open the catalog to page 6
A Hamilton Medical Company The medinCNO® is a CPAP driver that can be used in combination with the Medijet® nCPAP generator to administer CPAP therapy. The role of the medinCNO® in this CPAP system is to provide the necessary, possibly oxygen-enriched drive flow, which is fed to the nCPAP generator Medijet® via the tubes connected to the patient and is converted into CPAP pressure within the generator. An internal electronic blender combines the air and oxygen coming from an external source and administers the total volume so that the drive flow reaching the patient can be enriched with...
Open the catalog to page 7
Oxygen concentration: Pressure measurement: Push (inspiration support by the medinCNO®) Electronic shut-off valve Mechanical overpressure valve Operating Time medrn A Hamilton Medical Company Accuracy: ±1 L/min (in the working range) ±2 L/min (outside of the working range) Basis of measurement: DIN 1343 at 0°C, 1013.25 mbar and 0% air humidity (0/1013) Setting range: 21% to 100% (in flow working range) Measurement range: 21% to 100% Accuracy ± 3% (Vol.) MLF - 16 or OOM 102 measurement sensor Measurement range 0 mbar to 15 mbar - pressure graph Accuracy ±1.3 mbar Verification: Redundant...
Open the catalog to page 8
without difficulty. The medinCNO® does not contain any switch which disconnects it from the mains. Changes and modifications to the medinCNO® are not permitted without the permission of the manufacturer: see chapter 9.4 Repairs and an exchange of parts may only be made by trained, professional service personnel and only in accordance with the instructions in the Maintenance Manual, observing warnings in the manual and in these instructions for use. The medinCNO® and its power supply unit are not suitable for use in the direct vicinity of the patient. Only the patient tubes, the Medijet® and...
Open the catalog to page 9
Installation of the medinCNO® – Conditions Prior to start-up, the medinCNO® must be securely mounted. In doing so, the following conditions must be observed: 3.1 Figure 3-1: a) Brackets holding the medinCNO® in position; b) Ventilation slits; c) Overpressure valve; d) Knurled screws The medinCNO® must always be securely mounted before the device is started up. To do this, the two fixing brackets on the back of the device (Figure 3-1-a) are inserted into a standard rail (cross-section: 10 x 25 mm) and the brackets are then fixed in place manually using the knurled screws (Figure 3-1– d). It...
Open the catalog to page 10
Power supply Mains operation The power supply unit (REF 39-113) of the medinCNO® may only be connected to a power supply with the following properties: • Voltage: 100 – 240 V~ (alternating current) Strength of current: at least 1.1 A The power supply unit of the medinCNO® has a C8 connector for connection to the mains grid. The power line between the power supply unit of the medinCNO® and the mains grid must therefore meet these conditions: • Power supply side: plug appropriate for the country Device side: C7 plug in accordance with EN60320 Minimum rated voltage: the minimum rated voltage...
Open the catalog to page 11
Figure 3-2: Battery charge status displayed in the top left-hand area of the screen The charge status of the internal battery is displayed in the top left-hand area of the medinCNO® screen (Figure 3-2). A full battery is shown with a symbol with five bars. As soon as only two bars remain, the battery symbol is shown in red and the medinCNO® should be connected to the mains soon. As soon as there is only one bar left, the battery alarm is triggered. Attention: When the medinCNO® is running on battery power, an alarm is sounded when the internal battery is low. If this happens it is essential...
Open the catalog to page 12
In order to operate the medinCNO®, it must be supplied with air and oxygen from an external source that meets these conditions: Medicinal oxygen: - H2O ≤ 67 ppm (V/V) (free from condensate) H2O ≤ 870 ppm (V/V) and free from condensate! If as a result of the operating temperature it is possible that condensate may form despite a relatively low concentration of H2O in the air, then the concentration of H2O must be reduced accordingly. Figure 3-3: Water trap in the medinCNO® air inlet; a) Bolt for removing the condensate from the water trap The medinCNO® can be fitted with a water trap in the...
Open the catalog to page 13All Medin Medical Innovations GmbH catalogs and technical brochures
-
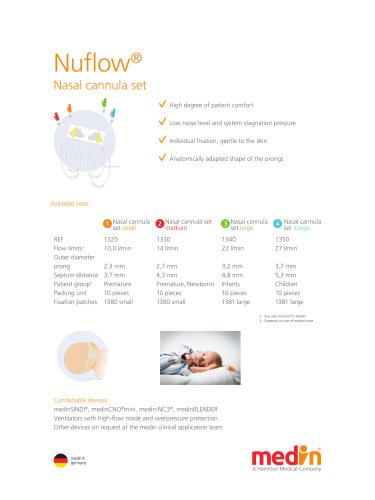
Nuflow®
2 Pages
-
Pb_1000_long
3 Pages
-
PB_medinBLENDER_1093_1094
2 Pages
-
OP_heated_circuits_all_languages
24 Pages
-
OP_Nuflow_all_languages
127 Pages
-
OP_Medijet
103 Pages
-
Pb_4000_long
3 Pages
-
OP_Miniflow
103 Pages
-
OP Bubble
2 Pages
-
medin Company Brochure
16 Pages
-
Processing
4 Pages
-
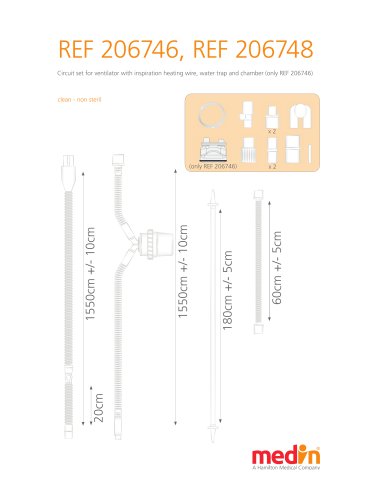
Pb_20674X_short
2 Pages
-
Pb_20674X_long
3 Pages
-
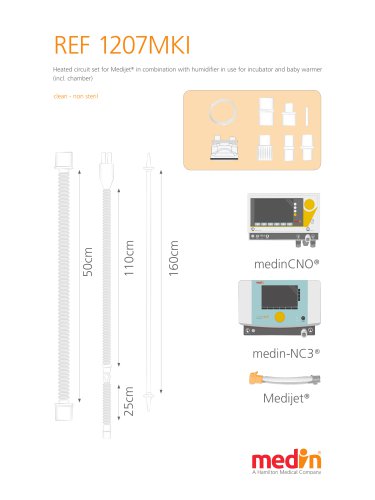
Pb_1207_long
2 Pages
-
PB_1207MKI
2 Pages
-
PB_1207
2 Pages
-
OP_Blender_Rev09_all languages
115 Pages
-
Nuflow® Nasal cannula set
2 Pages
-
Promo Sheet Nuflow
2 Pages
-
Order Information Nuflow
2 Pages
-
OP Pediflow all languages
65 Pages
-
OP_medinCNOmini_3080_EN_Rev03
64 Pages
-
OP_medinSINDI_1080_REV14
36 Pages
-
Guideline_for_Transport_Rev06
20 Pages
Archived catalogs
-
medin Company Brochure
16 Pages
-
Flyer_medin_Rev04
2 Pages
-
Prospect_products_medin_Rev09
20 Pages
-
medinCNO®mini
4 Pages
-
MEDINsindi
2 Pages
-
Short_Application_Medijet_
2 Pages
-
Catalogue Pediflow®
4 Pages
-
Prospect_nasal_canula
4 Pages
-
Prospect_Miniflow_Bubble
4 Pages
-
Prospect_medinCNO
4 Pages
-
Medin_Catalog
16 Pages
-
Flyer_reuseable_bonnets
1 Pages
-
user_manual_medinSINDI
36 Pages
-
OP_Blender
18 Pages
-
OP_Circuits_heated
32 Pages
-
user_manual_medinCNO
59 Pages
-
OP_Miniflow_4000
96 Pages
-
OP_Medijet_1010_1020
4 Pages
-
OP_Medijet_1000
104 Pages
-
OP_Circuits_unheated
32 Pages
-
technical_data_medinSINDI
1 Pages
-
products_medin
16 Pages