カタログの抜粋
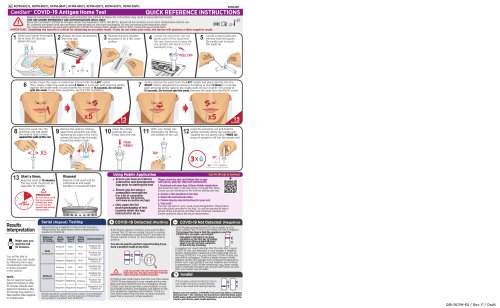
ENGLISH CareStartm COVID-19 Antigen Home Test QUICK REFERENCE INSTRUCTIONS Read all instructions carefully before performing the test. Failure to follow the instructions may result in inaccurate test results. /A FOR USE UNDER EMERGENCY USE AUTHORIZATION (EUA) ONLY. Store the CareStart™ COVID-19 Antigen Home Test between 1-30°C (34-86°F). Ensure all kit contents are at room temperature before use. Kit contents are stable until the expiration date printed on the outer packaging. Do not use beyond the expiration date. For more information on expiration dating for COVID-19 antigen tests, please refer to http://www.fda.gov/covid-tests. IMPORTANT: Swabbing the nostrils is critical for obtaining an accurate result. If you do not swab your nose, the device will produce a false negative result. Remove the test cassette and place it on a flat, clean surface, Locate the extraction vial and gently peel off the aluminum foil seal, being sure to keep the vial upright and place it in the packaging tray. against the inside walls of your nostrils for a total of 15 seconds. Do not just spin the swab. If you have questions, see the CDC Guidelines. Place the swab into the extraction vial and rotate the swab at least 5 times against the walls of the vial. Remove the swab by rotating against the extraction vial while squeezing the sides of the vial to release the liquid from the swab. Discard the swab in trash. 10 Close the vial by pushing the cap firmly onto the vial. 11 With your finger, mix thoroughly by flicking the bottom of the vial. 12 Invert the extraction vial and hold the sample vertically above the sample well. Squeeze the vial gently. Allow THREE (3) drops of sample to fall into the sample well. 13 Start a timer. Read the result at 10 minutes. The test result should not be read after 15 minutes. Disposal Dispose of all used test kit components and swab samples in household trash. Using Mobile Application Scan the QR code to download ► Ensure you have an internet connection and download the App prior to starting the test ► Ensure you are using a compatible smartphone. (For a list of compatible smartphone OS systems, visit www.accessbio.net/app) ► Only open the foil pouch packaging of test cassette when the App instructed to do so. Please start the test and follow the in-app self-paced, step-by-step test instructions. 1. Download and open App, Othena Mobile Application Download the App on the App Store or Google Play Store. Ensure you are connected to the internet during your test. 2. Answer a few questions in the App3. Watch the instructional video.4. Follow step-by-step instructions for your test.5. Test result The App will assist in your visual result interpretation. Please follow the instructions provided in the App. You will be required to take a picture of the test device, and then look at the test cassette and answer questions about the result interpretation. ResultsInterpretation Serial (Repeat) Testing COVID-19 Detected (Positive) COVID-19 Not Detected (Negative) Repeat testing is needed to improve test accuracy. Please follow the table below when interpreting test results for COVID-19. Make sure you wait the full 10 minutes. You will be able to Interpret your test results by following the in-app interpretation instructions or those provided in this section. NOTE: Do not read test results before 10 minutes or after 15 minutes. Results read before 10 minutes or after 15 minutes may lead to a false positive, false negative, or invalid result. If the Purple-colored Control (C) line and the Blue-colored Test (T) line are visible, the test is positive. Any faint visible Blue-colored Test (T) line with the Purple-colored Control (C) line should be read as positive. You do not need to perform repeattesting if you have a positive result at any time. If the Purple-colored Control (C) line is visible, but the Blue-colored Test (T) line is not visible, the test is negative. To increase the chance that the negative COVID-19 is accurate, you should: -Test again in 48 hours if you have symptoms on the first day of testing. -Test 2 more times at least 48 hours apart if you do not have symptoms on the first day of testing. result for A Positive test result means that the virus that causes COVID-19 was detected in your sample and it is very likely you have COVID-19 and are contagious. Please contact your doctor/primary care physician or your local health authority immediately and adhere to the local guidelines regarding self-isolation. There is a very small chance that this test can give a positive result that is incorrect (a false positive). A negative test result indicates that the virus that causes COVID-19 was not detected in your sample. A negative result is presumptive, meaning it is not certain that you do not have COVID-19. You may still have COVID-19 and you may still be contagious. There is a higher chance of false negative results with antigen tests compared to laboratory-based tests such as PCR. If you test negative and continue to experience COVID-19-like symptoms, (e.g., fever, cough, and/or shortness of breath) you should seek follow up care with your health care provider. If the Purple-colored Control (C) line is not visible, the test is invalid. Re-test with a new swab and new test device. Report your test result(s) at MakeMyTestCount.Org (https://makemy testcount.org) - this voluntary and anonymous reporting helps public health teams understand COVID-19 spread in your area and across the country and informs public health decisions. Status on First Day of Testing Results should be considered in the context of an individual’s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19.
カタログの1ページ目を開く
The CareStart™ COVID-19 Antigen Home Test is a lateral flow immunoassay device intended for the qualitative detection of nucleocapsid protein antigen from the SARS-CoV-2 virus. This test is authorized for non-prescription home use with self-collected anterior nasal (nares) swab samples from individuals aged 14 years or older or adult collected anterior nasal (nares) swab samples from individuals aged 2 years or older. This test is authorized for individuals with symptoms of COVID-19 within the first 7 days of symptom onset when tested at least twice over three days with at least 48 hours...
カタログの2ページ目を開くAccess Bioのすべてのカタログと技術パンフレット
-
CareStartTM Flu A&B Plus
23 ページ