カタログの抜粋
Leading Technology in Fluid-stable Reagents from DiaSys · · · · · · · · High Performance in Renal Diagnostics Over 25 years of experience in development and production of clinical chemistry tests Premium service supply in technics, applications and after sales Quality products made in Germany High performance, ready-to-use reagents with minimized interferences, long shelf life, on-board stability and traceability to international references Perfectly matched fluid-stable reagents, calibrators and controls High grade raw materials from traceable origin Processes and resources certified according to ISO 13485, ISO 9001, fulfilling highest quality standards Sustainable processes and products preserve the environment DiaSys offers reagent kits for manual and automated use plus the appropriate calibrators and controls. Detailed information about the Cystatin C test is available on our website www.diasys-diagnostics.com/products/reagents and in our product catalogue. Climate-neutral print (Carbon neutral) ID: DE-626-777935 DiaSys Diagnostic Systems GmbH Alte Strasse 9 65558 Holzheim Germany Printed on FSC-certified paper Early. Ageless. Powerful. Overcoming Diagnostic
カタログの1ページ目を開く
Although the occurrence of kidney diseases is very common and disease progression is harmful, nephropathies are well treatable, if detected early. Since kidney diseases develop slowly and at least in the beginning painless, the majority of individuals with early stages of chronic kidney disease (CKD) remain undiagnosed. Therefore, early detection of renal insufficiency by a sensitive marker as Cystatin C is of increasing importance to avoid the irreversible condition of renal failure and to initiate subsequent treatment. Method comparison vs nephelometric method Method comparison vs...
カタログの2ページ目を開く
Although the occurrence of kidney diseases is very common and disease progression is harmful, nephropathies are well treatable, if detected early. Since kidney diseases develop slowly and at least in the beginning painless, the majority of individuals with early stages of chronic kidney disease (CKD) remain undiagnosed. Therefore, early detection of renal insufficiency by a sensitive marker as Cystatin C is of increasing importance to avoid the irreversible condition of renal failure and to initiate subsequent treatment. Method comparison vs nephelometric method Method comparison vs...
カタログの3ページ目を開く
Leading Technology in Fluid-stable Reagents from DiaSys · · · · · · · · High Performance in Renal Diagnostics Over 25 years of experience in development and production of clinical chemistry tests Premium service supply in technics, applications and after sales Quality products made in Germany High performance, ready-to-use reagents with minimized interferences, long shelf life, on-board stability and traceability to international references Perfectly matched fluid-stable reagents, calibrators and controls High grade raw materials from traceable origin Processes and resources certified...
カタログの4ページ目を開くDiaSys Diagnostic Systems GmbHのすべてのカタログと技術パンフレット
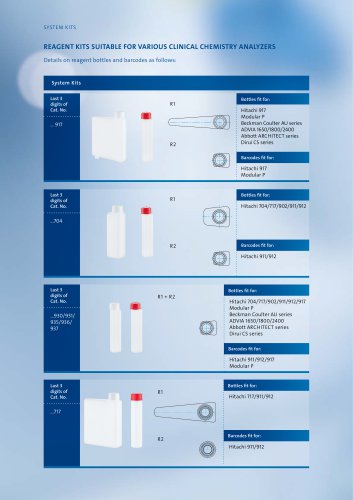
-
respons®910
4 ページ
-
respons®910VET
3 ページ
-
DiaSys
104 ページ
-
HbA1c net FS
6 ページ
-
InnovaStar®
6 ページ
-
respons®920
4 ページ
-
Electrolytes
4 ページ
-
D-Dimer FS
4 ページ
-
Iron Metabolism
4 ページ
-
oneHbA1c FS
4 ページ
-
Homocysteine FS
4 ページ
-
Immunoturbidimetry
4 ページ
-
Metabolic Syndrome
4 ページ
-
CRP U-hs
4 ページ
-
Lactate FS
2 ページ
-
Prealbumin FS
2 ページ
-
LP-PLA2
4 ページ
-
SensoStar GL30 touch
2 ページ
-
Ethanol FS
2 ページ
-
CRP FS
4 ページ
-
Creatinine PAP FS
2 ページ
-
CK-MB FS
2 ページ
-
Cholinesterase FS
2 ページ
-
Bicarbonate FS
2 ページ
-
SensoStar GLHsix
2 ページ