カタログの抜粋

EC DECLARATION OF CONFORMITY AT UYGUNLUK BEYANI Document Code | Dokuman Kodu Release Date | Yayın tarihi Revision Date | Revizyon tarihi DECLARATION AS THE MANUFACTURER, WE DECLARE THAT THE MEDICAL DEVICES WHICH ARE LISTED IN ANNEX-1, MEET THE REQUIREMENTS OF THE 2017/745 MEDICAL DEVICE REGULATIONS AND THE RELEVANT NATIONAL REQUIREMENTS BEYAN ÜRETİCİ OLARAK EK-1'DE BELİRTİLEN TIBBİ CİHAZLARIN 2017/745 TIBBİ CİHAZ YÖNETMELİĞİ VE İLGİLİ ULUSAL GEREKLİLİKLERİ KARŞILAŞTIĞINI BEYAN EDERİZ. CE MARKING START DATE CE MARKALAMA BAŞLANGIÇ TARİHİ PLACE, DATE | YER, TARİH ISTANBUL, TURKEY İSTANBUL,...
カタログの2ページ目を開くOncu Dental AS - Dentacのすべてのカタログと技術パンフレット
-
T-RESTO PRODUCT CATALOG
16 ページ
-
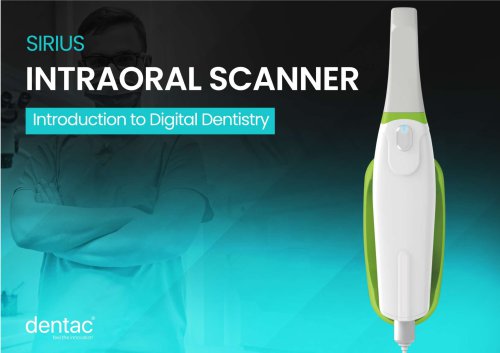
Sirius
6 ページ
-
Catalogue 2024
48 ページ
-
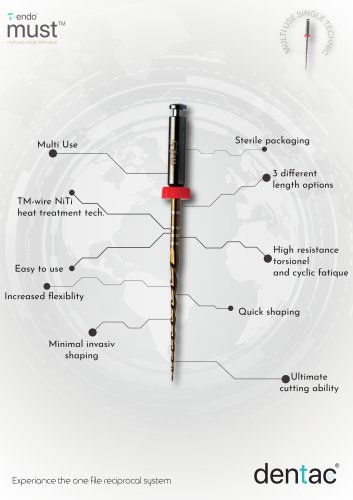
must Brochure
6 ページ
商品比較を空にする