カタログの抜粋

RAPID RHINO™ NASASTENT™ Introducing a structural nasal dressing designed to completely dissolve, not fragment Trimmable and resilient for ease of placement, with no special storage conditions required Dissolvable Nasal Dressin
カタログの1ページ目を開く
NASASTENT™ nasal dressing The resiliency of NASASTENT nasal dressing allows for ease of placement that conforms to patient anatomy and remains where placed. NASASTENT nasal dressing is a dissolvable intranasal splint made from plant-based CarboxyMethyl Cellulose (CMC). As it absorbs nasal fluids, it turns into a hydrocolloidal gel that naturally drains from the nasal cavity. Capable of absorbing up to 16 times its weight, NASASTENT nasal dressing is designed to be trimmable and resilient for ease of placement while conforming to patient anatomy, minimize bleeding and edema, and prevent...
カタログの2ページ目を開く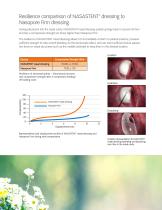
Resilience comparison of NASASTENT™ dressing to Nasopore Firm dressing During placement into the nasal cavity, NASASTENT nasal dressing quickly springs back to recover its form and has a compressive strength six times higher than Nasopore Firm.1 The resilience of NASASTENT nasal dressing allows it to immediately conform to patient anatomy, possess sufficient strength to help control bleeding via the tamponade effect, and can exert sufficient tissue separation force on nasal structures (such as the middle turbinate) to keep them in the desired location. Location Device NASASTENT nasal...
カタログの3ページ目を開く
Turbinate support load Instranasal splints need to provide sufficient support to tissue compromised by surgery and/or trauma, such as to prevent the lateralization of the middle turbinate.1 Tissue separation force (N) NASASTENT nasal dressing Nasopore Firm Nasopore Firm Double-Fold (2) 8 9 Simulated nasal cavity gap (mm) Tissue Separation Force of NASASTENT nasal dressing and Nasopore Firm after being placed in simulated nasal cavity gaps ranging from 7 to 10mm. NASASTENT nasal dressing provides significantly higher separation forces compared to Nasopore Firm over all nasal cavity gap sizes...
カタログの4ページ目を開く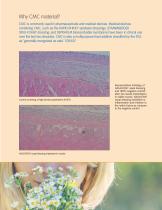
Why CMC material? CMC is commonly used in pharmaceuticals and medical devices. Medical devices containing CMC, such as the RAPID RHINO™ epistaxis dressings, STAMMBERGER SINU-FOAM™ dressing, and SEPRAFILM bioresorbable membrane have been in clinical use over the last two decades. CMC is also a multipurpose food additive classified by the FDA as “generally recognized as safe.” (GRAS)2 Control consisting of high density polyethylene (HDPE) NASASTENT nasal dressing implanted in muscle Representative histology of NASASTENT nasal dressing and HDPE (negative control) after two weeks implantation...
カタログの5ページ目を開く
Betre, Helawe and Delli-Santi, George. Performance Comparison of Nasal Dressings: Dissolvable NASASTENT™ vs. Fragmentable Nasopore Firm. Smith & Nephew. August 2014. 2. Cooper, Monica. CMC Considerations for 505(b)(2) Applications. FDA/CDER/OPS/ ONDQA AAPS Annual Meeting Washington, D.C. October 2011. Retreived from: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSeach.cfm?fr=182.1745 3. Valentine R, Wormald W, Sindwani R. Advances in Absorbable Biomaterials and Nasal Packing. Otolaryngology Clin N Am 2009; 42(5):813-828 4. Cowin A, McIntosh D, Wormald P. Healing of wounds...
カタログの6ページ目を開くSmith & Nephewのすべてのカタログと技術パンフレット
-
ANTHEM
40 ページ
-
BIRMINGHAM HIP
32 ページ
-
FREEDOM
16 ページ
-
SALTO TALARIS
48 ページ
-
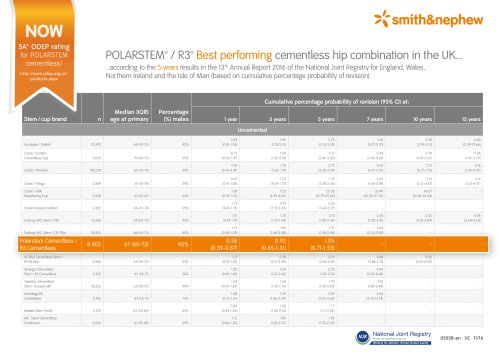
polarstem
28 ページ
カタログアーカイブ
-
Rediscover normal
4 ページ
-
TRIGEN™ INTERTAN
12 ページ
-
EVOS SMALL Resources
12 ページ
-
anthem
4 ページ
-
Ordering information
1 ページ
-
BST-CarGel ®
20 ページ
-
Electrosurgery
20 ページ
-
Powered Instruments
11 ページ
-
Shaver Systems
7 ページ
-
Knee
73 ページ
-
HIP
21 ページ