カタログの抜粋

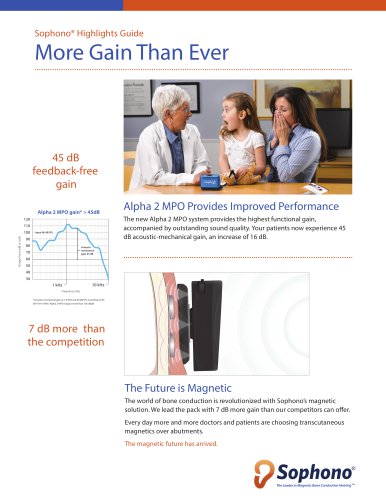
PRODUCT SPECIFICATION SOPHONO ALPHA 2 MPO PROCESSOR ® Abutment-Free Bone Conduction with up to 45dB of Feedback Free Gain Abutment-free hearing with no sacrifice in performance. Four Program Choices Now with a peak output of 115 dB, the Sophono Alpha 2 MPO gives your patients uncompromised sound with four programs, eight channels, and up to 320 hours battery life. Volume Control Compared with other transcutaneous hearing systems: §§ Up to 7dB more feedback free gain compared to other transcutaneous devices. Symmetrical Form Factor Sophono has the lowest processor profile, a sleek solution that may be hidden under the hair. Tamper-proof Battery Door The Alpha 2 MPO magnetic bone conduction system features a titanium encased magnetic implant, an external processor, and Attract magnetic spacers with variable strength to ensure patient comfort and secure fit. ® Feature Details Placement Headband Configuration Softband Configuration Abutment-Free Digital Signal Processor Data Logging Programs/Memories Microphone Direct Audio Input Retention Clip and Lanyard Auto Noise Reduction Auto Feedback Suppression Frequency Range Processor Power Supply Battery Life Peak Output Force Level at 90 dB SPL re 1µN Output Force Level at 60 dB SPL re 1µN Audible Warning Tones Colors Skinit Decal 3 Alpha 2 MPO processor Either side, with ergonomic grip design Yes Yes Yes Yes Yes 4 Two microphones in an isolated compartment with omni- and directional modalities Standard Europlug Standard Yes Yes 125 - 8000 Hz 16-band, 8-channel WDRC Type 13 zinc air battery Up to 320 hrs 115 dB Safety Line Anchor All Sophono products are specifically designed for amplification of sound force through the skin into the bone. We call this Transcutaneous Energy Transfer (TET ). ™ 105 dB Yes Anthracite, Brown, Champagne, Silver Gift card for one pair included Direct Audio Input Connector Programming Port Kohan, D. Implantable Auditory Devices. Presented at the Ultimate Colorado Midwinter Meeting; Vail, CO; Feb. 3, 2015 Sophono internal validation testing of battery life. Comparing published transcutaneous device specifications of acoustic-mechanical gain at 1.6 kHz and
カタログの1ページ目を開く
PRODUCT SPECIFICATION SOPHONO MAGNETIC IMPLANT ® Abutment-Free Bone Conduction with the Smallest Magnetic Implant No other implant system - abutment or magnetic, offers the low profile and stability of the Sophono device. Compared with other transcutaneous hearing systems: §§ Sophono boasts the smallest implant on the market (2.6 mm high). The implant is secure to the bone with 5 screws making it less likely to break, come loose or fall out during an impact. Our low profile implant follows the contour of the skull, ensuring a snug fit against the bone, resulting in a safer bone conduction...
カタログの2ページ目を開くSophonoのすべてのカタログと技術パンフレット
-
Safe & Sound
4 ページ