Catalog excerpts

For use with nasopharyngeal swab specimens For in vitro diagnostic use only CLIA complexity: MODERATE Rx Use only CareStartTM Flu A&B Plus Rapid Diagnostic Test for Detection of Influenza A and B Antigen Package Insert (Instructions for Use)
Open the catalog to page 1
Rapid Test for the Detection of Influenza A and B Antigen CareStartTM Flu A&B Plus Table of Contents
Open the catalog to page 2
Rapid Test for the Detection of Influenza A and B Antigen CareStartTM Flu A&B Plus Intended Use / Indications for Use The CareStartTM Flu A&B Plus is an in vitro rapid immunochromatographic assay for the qualitative detection of influenza virus type A and B nucleoprotein antigens directly from nasopharyngeal swab specimens of symptomatic patients. The test is intended for use as an aid in the rapid differential diagnosis of acute influenza type A and B viral infections. This test is intended to distinguish between influenza type A and/or B virus in a single test. This test is not intended...
Open the catalog to page 3
Rapid Test for the Detection of Influenza A and B Antigen CareStartTM Flu A&B Plus Test Principle and Summary The CareStartTM Flu A&B Plus test is an immunochromatographic assay for the detection of extracted influenza type A and B virus nucleoprotein antigens in nasopharyngeal specimens. Nasopharyngeal swabs require a sample preparation step in which the sample is eluted and washed off into the extraction buffer solution. Extracted swab sample is added to the sample well of the test device to initiate the test. When the swab sample migrates in the test strip, influenza A or B viral...
Open the catalog to page 4
Rapid Test for the Detection of Influenza A and B Antigen The following materials are needed but not provided: • Pair of gloves • Timer / Pen • Biohazard or sharps container Hazard and Precautionary Statements Hazardous codes Code Descriptions Code Descriptions H315v Causes skin irritation H335 May cause respiratory Precautionary codes Code Descriptions dust/fume/gas/mist/vapors/spray P264 Wash hands thoroughly after P273 Avoid release to the environment P270 Do not eat, drink or smoke when using this product gloves/protective clothing/eye protection/face protection P315 Get immediate...
Open the catalog to page 5
Rapid Test for the Detection of Influenza A and B Antigen • Other commercial controls have not been validated with this test and are not recommended. • If infection with a novel influenza virus is suspected based on current clinical and epidemiological screening criteria recommended by public health authorities, specimens should be collected with appropriate infection control precautions for novel virulent influenza viruses and sent to state or local health department for testing. Viral culture should not be attempted in these cases unless a BSL 3+ facility is available to received and...
Open the catalog to page 6
Rapid Test for the Detection of Influenza A and B Antigen CareStartTM Flu A&B Plus Quality Control Internal Quality Control: The CareStart™ Flu A&B Plus contains a built-in internal procedural control that is included in the test device. A purple-colored line appearing in the control region “C” is designed as an internal procedural control. The appearance of a purple procedural control line indicates that sufficient flow has occurred, and the functional integrity of the test device has been maintained. If the purple procedural control line does not develop at 10 minutes, the test result is...
Open the catalog to page 7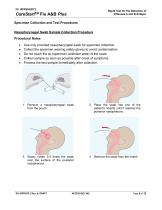
Rapid Test for the Detection of Influenza A and B Antigen CareStartTM Flu A&B Plus Specimen Collection and Test Procedures Nasopharyngeal Swab Sample Collection Procedure Procedural Notes • • • • • Use only provided nasopharyngeal swab for specimen collection. Collect the specimen wearing safety gloves to avoid contamination. Do not touch the tip (specimen collection area) of the swab. Collect sample as soon as possible after onset of symptoms. Process the test sample immediately after collection. 1. Remove a nasopharyngeal swab from the pouch. 2. Place the swab into one of the patient’s...
Open the catalog to page 8
Rapid Test for the Detection of Influenza A and B Antigen CareStartTM Flu A&B Plus Test Procedures Procedural Notes • • • • Allow test device, reagents, specimens, and/or controls to equilibrate to room temperature (15~30°C) prior to testing. Remove the CareStart™ Flu A&B Plus test device and extraction vial from its foil pouch immediately before testing. The CareStart™ Flu A&B Plus kit IS INTENDED to be used only with nasopharyngeal swab specimens. The CareStart™ Flu A&B Plus kit IS NOT INTENDED for testing liquid samples such as wash or aspirate samples or swabs in transport media as...
Open the catalog to page 9
Rapid Test for the Detection of Influenza A and B Antigen 9. Mix thoroughly by flicking the bottom of the tube. 10. Invert the extraction vial and hold the sample vertically above the sample well. Squeeze the vial gently. Allow three (3) drops of sample to fall into the sample well. 11. Read and interpret the test result after 10 minutes. The test result should not be read and interpreted after 15 minutes.
Open the catalog to page 10
Rapid Test for the Detection of Influenza A and B Antigen CareStartTM Flu A&B Plus Interpretation of Results NOTE: The test result should not be read and interpreted after 15 minutes. Do not interpret the result using any instruments. Influenza Antigen Positive: Influenza A infection: two distinct colored lines appear. One purple-colored line next to “C” and one red-colored line C next to “A” indicates influenza A positive result. B Influenza B infection: two distinct colored lines appear. One purple-colored line next to “C” and one blue-colored line C next to “B” indicates influenza B...
Open the catalog to page 11
Rapid Test for the Detection of Influenza A and B Antigen One purple-colored line only next to "C” indicates a negative result. If the purple-colored line in the control region "C” is not visible, the result is invalid. Re-run the test one time using the remaining specimen in the extraction vial if an invalid result is obtained during initial testing. 1. This test will indicate the presence of influenza virus type A and B nucleoprotein antigens in the specimen from both viable and non-viable influenza virus. 2. The detection of viral antigen is dependent upon proper specimen collection,...
Open the catalog to page 12All Access Bio catalogs and technical brochures
-
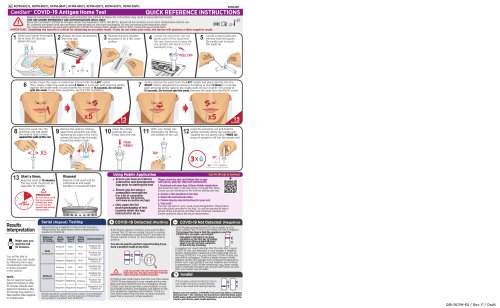
COVID-19 Antigen Home Test
10 Pages