Website:
ADIN Dental Implant Systems Ltd.
Website:
ADIN Dental Implant Systems Ltd.
Catalog excerpts

DENTAL IMPLANT SYSTEMS LTD Implant System
Open the catalog to page 1
About ADIN Adin Dental Implant Systems Ltd., designs, manufactures and markets state of the art, technologically advanced dental implant solutions. For more than 20 years, Adin has provided dentists and dental technicians with innovative solutions and advanced knowledge into the field of implant dentistry. The Adin plant and headquarters is located in northern Israel and employs highly skilled staff each of whom play a significant role in Adin’s growing success. Adin highly values each hard working employee and attributes its growing success to their continuous dedication. Over the years,...
Open the catalog to page 2
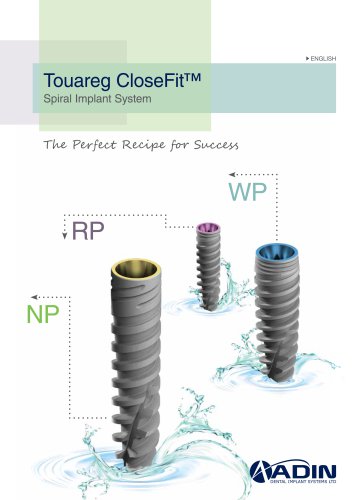
Touareg CloseFitTM The Touareg CloseFit™ spiral implant system offers a unique, strong and solid conical-hex connection, enabling implants to be used in all indications and regions, in the lower and the upper jaw. The CloseFit™ conical-hex connection minimizes micro movements between the implant and abutment. The Touareg CloseFit™ spiral implant system is comprised of a tapered core implant with a spiral tap, promoting increased immediate stability. Unlike conventional self-tapping implants, which scrape the bone away as they tap, Touareg Closefit™ implants slice through the bone, thanks to...
Open the catalog to page 3
Narrow Platform Implant System The Touareg CloseFit™ NP is a uniquely designed implant with an especially nararow body and a 3.0mm prosthetic platform diameter. The strong and solid CloseFit™ conical-hex connection enables this implant to be used in the lower and upper jaw. With high quality and narrow platform switching, the Touareg CloseFit™ NP 3.0mm has proven to have a very high fatigue strength and best comparative results, what makes it the most successful and leading narrow platform implant. Touareg™ NP 3.0mmD Product Assortment (The full components list can be found in Adin’s...
Open the catalog to page 4
Touareg CloseFitTM RP 3.5mmD The Touareg™ RP is a 3.5mmD platform implant that features the unique, strong and solid CloseFit™ conical-hex connection with the OsseoFix™ surface treatment. The Touareg CloseFit™ RP can be used in all indications, in all regions on the lower and upper jaw. Regular Platform Implant System (The full components list can be found in ADIN’s Product Catalog) RP Slim Healing Abutment 3.5mmD x 3mmL RP Slim Healing Abutment 3.5mmD x 4mmL RP Conical Healing Abutment 4.5mmD x 3mmL RP Conical Healing Abutment 4.5mmD x 4mmL RP Straight Slim Abutment - No End Line RP...
Open the catalog to page 5
Touareg CloseFitTM WP 4.3mm/5.0mmD The Touareg™ WP is a uniquely designed wide platform implant available in 4.3mm and 5.0mm platform diameter. The strong and solid CloseFit™ conical-hex connection enables these implants to be used in all indications in all regions on the lower and upper jaw. The collars of the WP 4.3mmD and 5.0mmD implants are backbeveled. Wide Platform Implant System Touareg™ WP 4.3mmD / 5.0mmD Product Assortment (The full components list can be found in ADIN’s Product Catalog) WP Slim Healing Abutment 4mmD x 3mmL WP Slim Healing Abutment 4mmD X 4mmL WP Conical Healing...
Open the catalog to page 6
High Fatigue Strength High Fatigue Strength - TouaregTM NP Fatigue strength is the maximum force an implant / abutment assembly can survive for at least five million cycles. Adin uses a protocol for fatigue strength testing according to ISO 14801. Fatigue strength results are presented beside. Adin’s Touareg CloseFit™ NP 3.0mmD implant achieved very high fatigue strength results of 260 N and 380 N, whilst NobelActive™ 3.0 achieved 160 N and Astra Tech 3.0 achieved 130 N. Note: Nobel and Astra data was taken directly from Nobel Biocare’s NobelActive 3.0 brochure. Astra Tech and OsseoSpeed...
Open the catalog to page 7
ADIN products comply with the standards set by the FDA and other regulatory agencies. All ADIN products are CE-marked in accordance with the Council Directive 93/42/EEC and Amendment 2007/47/EC. ADIN complies with ISO13485:2012 and the Canadian Medical Devices Conformity Assessment System (CMDCAS). Product availability may vary between countries. For more information, please contact your local ADIN office. Industrial Park Allon Tavor, POB 1128, Afula 1811101, Israel | T. +972-4-6426732 | info@adin-implants.com
Open the catalog to page 8All ADIN Dental Implant Systems Ltd. catalogs and technical brochures
-
Patient Information Brochure
5 Pages
-
TMA
8 Pages
-
Standard Internal Hex
8 Pages
-
Harpoon ™ 1
4 Pages
-
Surgical Kits
3 Pages
-
Scientific Studies Abstracts
8 Pages
-
Surgical DLC Drills
4 Pages
-
Catalog
74 Pages
Archived catalogs
-
Patient Information Brochure
6 Pages