 Website:
Alcon
Website:
Alcon
Catalog excerpts

40-500-096-002 PRODUCT INFORMATION Alcon Laboratories, Inc. delivery system
Open the catalog to page 1
PRODUCT INFORMATION Alcon Laboratories, Inc. delivery system STERILE UV and Blue Light Filtering Acrylic Foldable Single-Piece Posterior Chamber Lenses with the AcrySert® C Delivery System CAUTION: Federal (USA) law restricts this device to the sale by or on the order of a physician. Model Characteristics Chart Model Optic Type Haptic Angle Anterior Asymmetric Biconvex PHYSICAL CHARACTERISTICS All dimensions in millimeters DESCRIPTION AcrySof® IQ UV and blue light filtering acrylic foldable single-piece posterior chamber lenses are optical implants for the replacement of the human...
Open the catalog to page 2
UV-light filtering, the AcrySof® IQ IOL reduces transmittance of blue light wavelengths from 62% at 400 nm to 23% at 475 nm (see Table 1). The optical portion consists of a high refractive index soft acrylic material. This material is capable of being folded prior to insertion. The lens gently unfolds to a full-size lens body following implantation. The lens has a biconvex optic with supporting haptics. The posterior aspheric surface of the AcrySof® IQ Model SN6CWS IOL is designed with negative spherical aberration to compensate for the positive spherical aberration of an average cornea....
Open the catalog to page 3
The image quality of the Model SN6CWS was characterized by measuring modulation transfer function (MTF) in a model eye described in ISO 11979-2. The ISO 11979-2 requires MTF measurements using only a 3-mm aperture. In addition, the image quality of the Model SN6CWS was characterized by measuring MTF in a model eye that utilized a simulated cornea exhibiting typical adult human spherical aberration. Using the modified model eye, MTF measurements were made using both 3-mm, and 5-mm apertures. MODE OF ACTION AcrySof® IQ posterior chamber intraocular lenses are intended to be positioned in the...
Open the catalog to page 4
11. The clinical study of the AcrySof® Natural Single-Piece Lens (referenced in Tables 2 through 5) was conducted with the lens intended for implantation in the capsular bag only. There is no clinical data to demonstrate its safety and effectiveness for placement in the ciliary sulcus. It is recommended that viscoelastic be removed from the eye at the close of surgery with emphasis on the space between the posterior capsule and lens. This may be accomplished by gently depressing the IOL optic posteriorly with the I/A tip and using standard irrigation/aspiration techniques to remove the...
Open the catalog to page 5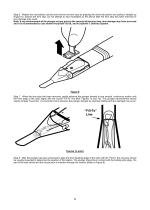
Step 6. Retract the viscoelastic cannula and remove the lens stop by gripping the arrow tab portion and pulling it straight up (Figure 6). Discard the lens stop. Do not attempt to add viscoelastic to the device after the lens stop has been removed or lens damage may result. Note: If any advancement of the plunger occurs prior to the removal of the lens stop, lens damage may have occurred and it is recommended to use another AcrySof® IQ IOL and AcrySert® C Delivery System. Figure 6 Step 7. When the lens stop has been removed, gently advance the plunger forward in one smooth, continuous...
Open the catalog to page 6
Figure 8 Step 9. After confirming the lens is properly positioned and the haptics are folded properly, proceed with lens implantation by inserting the nozzle tip through the incision and positioning the nozzle tip at the anterior capsule opening. Step 10. Gently advance the plunger in one smooth, continuous motion. Do not advance the plunger too fast or lens damage may occur. It is recommended that delivery of the lens from the visual inspection position should take at least 5 seconds. As the lens begins to exit the nozzle tip, place the leading haptic into the capsular bag and continue to...
Open the catalog to page 7
are Black. The control (SA30AL) subject population was 96.6% Caucasian, 2% Black and 1.4% other. The mean age for the total population receiving the Model SB30AL in at least the first operative eye is 72.9 years. Similarly, the mean age for the total population receiving the Model SA30AL (control) is 71.9 years. AcrySof® Natural Single-Piece Lens Model SB30AL Visual Acuity A summary of visual acuity achieved at a minimum of one year postoperatively among subjects who did not have preoperative ocular pathology, abnormal corneas, or macular degeneration at any time (Best Case) is presented in...
Open the catalog to page 8
Table 3b Best Corrected Visual Acuity in the Overall Patient Population at a Minimum of One Year Postoperatively, AcrySof® IOL SA30AL control Acuity > 20/40 to < 20/80 AcrySof® Natural Single-Piece Lens Model SB30AL Cumulative Adverse Events The cumulative rates of these adverse events up to and including a minimum of a one year postoperative period for the AcrySof® Natural Single-Piece Lens Model SB30AL and the Model SA30AL patients are shown in Table 4. There were no statistically significant differences between the Model SB30AL and the Model SA30AL for the proportion of subjects...
Open the catalog to page 9
Table 5 Persistent Adverse Events at a Minimum of One Year Postoperatively AcrySof® Natural IOL SB30AL and SA30AL control SB30AL (N= 138) N Type of Adverse Event Persistent Corneal Edema Macular Edema Raised IOP Requiring Treatment *p-values from Fisher’s Exact Test comparing Model SB30AL to Model SA30AL. AcrySof® Natural Single-Piece Lens Model SB30AL Color Perception Color perception testing using the Farnsworth D-15 Panel Test was conducted at the 120 to 180 day postoperative period. Of the 109 subjects with normal color vision implanted with a Model SB30AL in the first operative eye and...
Open the catalog to page 10
Mean Spherical Aberration Overall and by Age Group 90-120 Days after 2nd Eye Implant ■ AcrySof® IQIOL □ Control • Denotes statistical significance between lenses (p<0.0001) AcrySof® IQ Lens - Distance Visual Acuity The AcrySof® IQ lens and the control lens provided clinically similar postoperative visual acuity. Monocular visual acuity results are presented in Figures 11 and 12. LogMAR BCVA Days Postoperative • Quantitative Lens Model Main Effect (p=0.0214) LogMAR UCVA 1-2 Days 7-14 Days 30-60 Days 90-120 Days Days Postoperative Differences are not statistically significant
Open the catalog to page 11All Alcon catalogs and technical brochures
-
PUREPOINT
2 Pages
-
Vektor
2 Pages
-
NEGENUITY
2 Pages
-
CONSTELLATION®
4 Pages
-
MODEL MA50BM
11 Pages
-
Maintaining Good Eye Health
3 Pages
-
The LenSx® Laser System
12 Pages
-
CZ70BD
7 Pages
-
MODEL SN6AT3, SN6AT4, SN6AT5
23 Pages
-
MODEL SN60AT
9 Pages
-
MODEL SN6AD1
26 Pages
-
MODEL MA30AC
11 Pages
-
MODEL MN60AC
13 Pages
-
MODEL SN60WF
15 Pages
-
NAPHCON A
1 Pages
-
PUREPOINT
6 Pages
-
CONSTELLATION® Vision System
10 Pages


























