
Catalog excerpts

Evolve to BioFlo Ports with Endexo Technology 96% LESS THROMBUS ACCUMULATION COMPARED TO BARD POWERPORT WITH CHRONOFLEX CATHETER (based on platelet count)1,2
Open the catalog to page 1
Proven to Reduce Thrombus Accumulation, In Vitro1 The BioFlo* Port is the first implantable port with Endexo* Technology, providing a catheter material more resistant to the in-vitro accumulation of blood components compared to non-coated port catheters (based on platelet count).1.2 Endexo Technology is a permanent and non-eluting polymer that is “blended” into the polyurethane from which the catheter is made. It is present throughout the catheter including the extraluminal, intraluminal and cut catheter surface of the tip. Endexo Technology remains present for the life of the catheter and...
Open the catalog to page 2
BioFlo Ports—Combining the Power of PASV with CT Rated Ports The Power of PASV VA The PASV* Valve Technology design automatically resists backflow, reducing blood reflux that could lead to catheter-related complications. BioFlo Power-Injectable Ports are indicated up to 5mL/sec and 300 psi and are MRIconditional—3 Tesla. Radiopaque under fluoroscopy Flexible, kink-resistant catheter material 1/2” Septum diameter for ease of port access PASV Valve Technology designed to resist backflow, maintain long-term patency, and provide a saline-only flushing option Plastic Port with PASV Valve Technol
Open the catalog to page 3
REDUCTION NET MAINTENANCE COST PER PORT TOTAL COST PER PATIENT ($) REDUCTION IN INADEQUATE BLOOD DRAW REDUCTION IN TROUBLESHOOTING TIME Inability to draw blood—11% Non-valved, 5.8% PASV Valve Technology Nursing time—1,545 minutes Non-valved, 750 minutes PASV Valve Technology Rate of occlusion—6.7% Non-Valved, 4.4% PASV Valve Technol
Open the catalog to page 4
PASV Valve Technology is designed to: Open with minimal pressure and automatically close after infusion Open for sampling and automatically close to resist pressure fluctuations that may cause blood reflux Remain closed during normal increases in central venous pressure to prevent blood reflux in the catheter tip
Open the catalog to page 5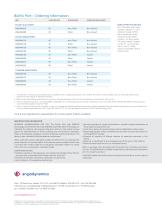
UPN CATHETER SIZE SUTURE HOLE PLASTIC DUAL PORTS H965440270 8F PLASTIC SINGLE PORTS H965440110 6F TITANIUM SINGLE PORTS H965440150 6F Non-Filled Filled Non-Valved Non-Valved Non-Filled Filled Non-Filled Filled Non-Filled Filled Non-Filled Filled Non-Valved Non-Valved Non-Valved Non-Valved Valved Valved Valved Valved BIOFLO PORT KITS INCLUDE: Port; Attachable single lumen catheter; Snaplocks (1); 18G Introducer needle; 0.038x 50cm J-guidewire; Valved introducer sheath; Metal tunneler; 17G Blunt needle; 22G Huber Needles: Straight (1), 90° (1); 12 mL Syringes: Slip (1), Luer...
Open the catalog to page 6All Angiodynamics catalogs and technical brochures
-
UniBlate
2 Pages
-
StarBurst SDE
2 Pages
-
StarBurst MRI
2 Pages
-
StarBurst XL
2 Pages
-
StarBurst RFA
16 Pages
-
Habib 4X
2 Pages
-
Model 1500X RF GENERATOR
4 Pages
-
BioFlo DuraMax
4 Pages
-
VenaCure EVLT
4 Pages
-
Triumph-1
2 Pages
-
TitanPort
2 Pages
-
LIFEPORT
2 Pages
-
StarBurst
16 Pages
-
Xcela Plus
4 Pages
-
StarBurst Xli-enhanced
2 Pages





















