Catalog excerpts

Minister*) dell* Salute DIREZIONE GENERALE DEI DISPOSITIVI MEDICI E DEL SERVIZIO FARMACEUTICO UFFICIO 3 DGDMF/III/P/I.5.I.e. 1/2020/833 HAVING REGARD to Directive 93/42/EEC concerning medical devices; HAVING REGARD to the Legislative Decree n. 46/97 and its following amendments implementing Directive 93/42/EEC; HAVING REGARD to the request with reference nr. 30542-A-21/05/2020, submitted by the Company ARDET DENTAL &MEDICAL DEVICES S.r.l., located in Via Larga 8, 20122 Milano (MI) Italy, Vat no.00641260960; WHEREAS the Company paid the fees required by Ministerial Decree January 16, 2019; HAVING REGARD to the official deeds; IT IS ATTESTED That, according to the Directive 93/42/EEC, the Company ARDET DENTAL & MEDICAL DEVICES S.r.l., located in Via Larga 8, 16015 Milano (MI) Italy, is the manufacturer and has marked CE as medical device the following products:
Open the catalog to page 1
The above mentioned products, according to the art. 4 of Directive 93/42/EEC, can freely circulate and can be placed on the market in Italy and all over the European Union. This document has been issued in a unique original version upon request of the manufacturer in order to export medical devices to Countries outside European Union It is not allowed any reproduction or publication of this document by paper, press, electronic base or websites. It is only allowed to show or to delivery it, upon request of the Customs or Health competent Authorities of the importing country. Executive...
Open the catalog to page 2All Ardet Dental & Medical Devices catalogs and technical brochures
-
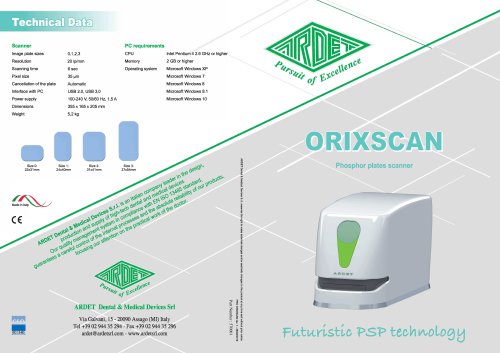
ORIXSCAN
2 Pages
-
ISO 13485-2016 Certificate
1 Pages
-
CE Certificate
6 Pages
-
ORIX HF Plus
2 Pages
-
ORIX HF Advantage
2 Pages
-
ORIX 70 New Edition DG
2 Pages
-
ORIX 70 New Edition
2 Pages
-
ORIX 70
2 Pages
-
IMAGE HD
2 Pages