
Catalog excerpts

RA - It’s Never too Early to Test
Open the catalog to page 1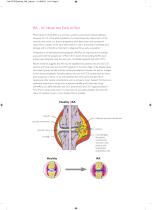
RA - It’s Never too Early to Test Rheumatoid Arthritis (RA) is a common, systemic autoimmune disease affecting between 0.5-1% of the adult population. It is characterised by inflammation of the synovial joints which can lead to progressive joint destruction and consequent impairment in quality of life. Early intervention is vital in preventing irreversible joint damage and it is therefore important to diagnose RA as early as possible.1 Antibodies to citrullinated proteins/peptides (ACPAs) are reported to be strongly associated with the progression of RA. In the routine clinical setting,...
Open the catalog to page 2
Anti-CCP and the Clinician Testing for Anti-CCP antibodies can provide the clinician with a number of important benefits: • Anti-CCP antibodies are highly specific for RA7 • Anti-CCP antibodies can be present many years before visit to clinician8 • In patients with undifferentiated RA the presence of anti-CCP antibodies can predict subsequent development to RA9,10 • Strong association between anti-CCP antibody levels and subsequent development of more severe disease (erosive)11,12 • New data to show that patients who are CCP+/RF+ or CCP+/RF- have a higher chance of developing erosive...
Open the catalog to page 3
More than 25 recent studies have compared the diagnostic performance of the Anti-CCP2 test versus Anti-CCP3/3. 1 and Anti-MCV (anti-mutated citrullinated vimentin) assays. Using a high stratified specificity approach (over 97%), the clinical sensitivity of the various ACPA tests and RF was reported as follows:7 Sensitivity at stratified specificity (%)
Open the catalog to page 4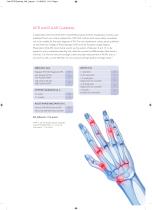
ACR and EULAR Guidelines In September 2010, the 2010 ACR / EULAR Rheumatoid Arthritis Classification Criteria were published. These new criteria replaced the 1987 ACR criteria which were widely considered not to be suitable for the early diagnosis of RA. The new classification criteria, jointly published by the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) recommend a point scoring system of between 0 and 10 to be applied to every individual presenting with definitive synovitis (undifferentiated inflammatory arthritis). For the first time, the...
Open the catalog to page 5
The new improved Axis-Shield Anti-CCP test (FCCP600) • Second generation peptide (CCP2) • Quantitative and/or qualitative protocols • Ready-to-use colour coded reagents • Break-apart wells • Increased dynamic range • Extended kit shelf-life References 1. Landewe RB, 2003; Arthritis Rhuem; 48:46-53, 2. Van Venrooij et al, 2008; Ann NY Acad Sci;ll43:268-285 3. Van der Helm-van Mil AH et al, 2005; Arthritis Res Ther;7:R949-R958 4. Pedersen M et al, 2006; Arthritis Res Ther;8:Rl33 5. Lopez-Longo F et al,2009;Arthritis Rheum;6l:4l9-424 6. van Dongen et al, 2007; Arthritis Rheum;56:l424-l432 7....
Open the catalog to page 6All Axis-Shield PLC catalogs and technical brochures
-
SyphScreen RPR (FRPR500)
2 Pages
-
HbA1c
4 Pages
-
Clinical Chemistry Anti-CCP
4 Pages
-
HOMOCYSTEINE
2 Pages
-
ACTIVE-B12
6 Pages









