
Catalog excerpts
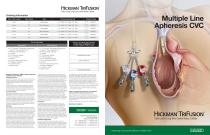
Ordering Information Tip to Cuff Length French Size HickmanTriFusion Triple Lumen Long-Term Central Venous Catheter Product Code Intermediate Microintroducer Intermediate Microintroducer Intermediate Microintroducer 0.8 - White / 0.9 - Blue / 0.8 - Red 0.8 - White / 0.9 - Blue / 0.8 - Red 0.8 - White / 0.9 - Blue / 0.8 - Red 0.8 - White / 0.9 - Blue / 0.8 - Red 1.0 - White / 1.0 - Blue / 1.0 - Red 1.0 - White /1.0 - Blue / 1.0 - Red Tray Components Intermediate Microintroducer Product and Packaging Do Not Contain Natural Rubber Latex • 12 F TriFusion® Polyurethane Catheter with SureCuff® Tissue Ingrowth Cuff • PTFE Peel-Apart Introducer •8 F Dilator • 18 G Introducer Needle • 70 cm Guidewire • Tunneler • Stat Lock® Securement Device • 2 Adhesive Dressings • 3 Connector Caps • Heparin Label • 12 F TriFusion® Polyurethane Catheter with SureCuff® Tissue Ingrowth Cuff • PTFE Peel-Apart Introducer •8 F Dilator •21 G Introducer Needle • 5 F Microintroducer • 45 cm Guidewire (0.018 in) • 120 cm Guidewire (0.038 in) • Tunneler • Stat Lock® Securement Device • 2 Adhesive Dressings • 3 Connector Caps • Heparin Label REPRESENTATIVE’S NAME PHYSICIAN’S SIGNATURE Hickman® TriFusion® Triple Lumen Long-Term Central Venous Catheter Indications for Use The Hickman® TriFusion® Triple Lumen Long-Term Central Venous Catheter is indicated for use in attaining short-term or long-term vascular access for intravenous infusion therapy and blood samplingviatheinternaljugularvein, external jugularvein, and subclavian vein. All Hickman® TriFusion® Catheters are designed for apheresis, and the administration of I.V. fluids, blood products, drugs, and parenteral nutrition solutions, as well as blood withdrawal. The Hickman® TriFusion® catheter incorporates three large, equal size lumens appropriate for apheresis procedures. Contraindications This device is contraindicated whenever: • The presence of device related infection, bacteremia, or septicemia is known or suspected. • The patient'sbody size is insufficient to accommodate the size of the implanted device. • The patient is known or is suspected to be allergic to materials contained in this device. • Severe chronic obstructive lung disease exists (percutaneous subclavian placement only). • Past irradiation ofprospective insertion site. • Previous episodes of venous thrombosis or vascular surgical procedures at the prospective placement site. • Local tissue factors will prevent proper device stabilization and/or access. Warnings • Percutaneous insertion of the catheter should be made into the axillary-subclavian vein at thejunction ofthe outer and mid-thirds of the clavicle lateral to the thoracic outlet. The catheter should not be inserted into the subclavian vein medially, because such placement may cause compression ofthe catheter between thefirst rib and clavicle which can lead to damage or fracture and embolization of the catheter.1 Fluoroscopic or radiographic confirmation of catheter tip placement should be helpful in demonstrating that the catheter is not being pinched by the first rib and clavicle.1 • Acetone and PEG-containing ointments can cause failure ofthis device and should not be used with polyurethane catheters. Chlorhexidine patches are the preferred alternative. • Cardiac arrhythmias may result if the guidewire is allowed to pass into the right atrium. • Close all clamps only in the center of the extension legs. Extensions may develop cuts or tears if subjected to excessive pulling or contact with rough edges. Repeated clamping near or on the luer lock connectors may cause tubing fatigue and possible disconnection. • Catheters should be implanted carefully to avoid any sharp or acute angles which could compromise the opening of the catheter lumens. • To prevent air embolism and/or blood loss, place thumb over the exposed orifice of the sheath introducer. • To avoid damage to vessels and viscus, infusion pressures should not exceed 25 psi (172 kPa). The use ofalOmlor larger syringe is recommended because smaller syringes generate more pressure than larger syringes. Note: Athreepound (13.3 Newton) forceon the plunger ofa3ml syringe generates pressure in excess of 30 psi (206 kPa) whereas the same three pound (13.3 Newton) force on the plunger ofalOml syringe generates lessthan 15 psi (103 kPa) ofpressure. • Accessories and components used in conjunction with this catheter should incorporate luer-lock adapters. • The heparin solution must be aspirated out of all lumens immediately prior to using the catheter to prevent systemicheparinization ofthe patient. • Failureto clamp extensions when not in use may lead to air embolism. • In the rare event of a leak, the catheter should be clamped immediately. Necessary remedial action must be taken prior to resuming the infusion procedure. • Do not resterilize the catheter or components by any method. The manufacturer will not be liable for any damages caused by reuse of the catheter or accessories. • Do not exceed flow rates of 140 ml per minute. Precautions • Carefully read and follow all instructions prior to use. • Only qualified healthcare practitioners should insert, manipulate and remove these devices. • Repeated over tightening of blood lines, syringes and caps will reduce connector life and could lead to potential connector failure. In case of damage, clamp the catheter between the patient and the damaged area with a smooth-edged, atraumatic clamp. • Sterile and non-pyrogenic only if packaging is not opened, damaged or broken. • Sterilized with Ethylene Oxide. • Single Patient UseOnly. • CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician. • Follow Universal Precautions when inserting and maintaining the catheter. • Follow all contraindications, warnings, cautions, precautionsand instructions for all infusates as specified by its manufacturer. Possible Complications The use of an indwelling central venous catheter provides an important means of venous access for critically ill patients; however, the potential exists for serious complications including the following: air embolism, bleeding, brachial plexusinjury, cardiac arrhythmia, cardiac tamponade, catheter or cuff erosion through skin, catheter embolism, catheteror cuffocclusion, catheterocclusion, damage or breakage dueto compression between the clavicle and first rib, catheter-related sepsis, endocarditis, exit site infection, exit site necrosis, extravasation, fibrin sheath formation, hematoma, hemothorax, hydrothorax, inflammation, necrosis or scarring of skin over implant area, intolerancereaction to implanted device, laceration of vessels or viscus, perforation of vessels or viscus, pneumothorax, spontaneous cathetertip malposition or retraction, thoracic duct injury, thromboembolism, venous thrombosis, ventricular thrombosis, vessel erosion, risks normally associated with local and general anesthesia, surgery, and post-operative recovery. 1 Aitken, D.R. and Minton, J.P. “The Pinch-Off Sign:AWarning of Impending Problems with Permanent Subclavian Catheters”, American JournalofSurgery, Vol. 148, Nov. 1984, pp. 683-638. Please consult package inserts for more detailed safety information and instructions for use. Bard, Advancing Lives and the Delivery of Health Care, Hickman, Statlock, and TriFusion are trademarks and/or registered trademarks of C. R. Bard, Inc., or an affiliate. All other trademarks are property oftheirrespectiveowners. Copyright©2015, C. R. Bard, Inc. All Rights Reserved. Illustrations byMikeAustin. Copyright©2015. All Rights Reserved. BPV/CVCA/1115/0001(1) rRAxrnm PERIPHERAL Multiple Line Apheresis CVC Hickman'TriFusion Advancing Lives and the Delivery of Health Care™
Open the catalog to page 1All Bard Medical catalogs and technical brochures
-
BOLSTER Clinical Study
2 Pages
-
TRUE® DILATATION
4 Pages
-
Atlas_Bro_LoRes
4 Pages
-
Sidekick-Usher
2 Pages
-
CTO-Family
10 Pages
-
MRI Safety
2 Pages
-
Catheter-Repair-Ki
2 Pages
-
HemoStarXK
2 Pages
-
GlidePath
8 Pages
-
POWERFLOW®
8 Pages
-
BARDEX I.C.
2 Pages
-
Closed Wound Drainage
12 Pages
-
SURESTEP
4 Pages
-
Ureteral Catheters Brochure
4 Pages
-
Wound Drainage Brochure
8 Pages
-
AGENTO
4 Pages
-
ISOSLEEVE
4 Pages
-
READYLINK
6 Pages
-
SOURCELINK
6 Pages
-
SourceCap
4 Pages
-
Biopsy Instruments
4 Pages
-
FIXT Suturing Device
4 Pages
-
ALYTE
4 Pages
-
ARCTICGEL
2 Pages
-
ARCTIC SUN® 5000
12 Pages
-
IAP Brochure
4 Pages
-
CRITICORE® Monitor
2 Pages
-
INLAY OPTIMA
4 Pages
-
INLAY
4 Pages
-
EXPAND212
4 Pages
-
U-NITE
2 Pages
-
NICORE? Nitinol Guidewire
2 Pages
-
Filiforms
4 Pages
-
UROFORCE
4 Pages
-
X-FORCE
8 Pages
-
AQUAGUIDE Brochure
4 Pages
-
ENDOBEAM
4 Pages
-
Temporary Pacing Electrodes
4 Pages
-
Nasogastric Tubes (NG)
4 Pages
-
STATLOCK Foley Brochure
2 Pages
-
Coude Advance Foley Tray
2 Pages
-
The ADVANCE Foley Tray
6 Pages
-
Lubri-Sil® I.C. Brochure
4 Pages
-
Coated Latex Foley Catheters
6 Pages
-
Specialty Foley Catheters
4 Pages
-
TOUCHLESS PLUS® Brochure
2 Pages
-
Urological Catheters
2 Pages



























































