Catalog excerpts

BAUMER Hydrogen Peroxide Sterilizer - PHB BAUMER Hydrogen Peroxide Sterilizer - PHB SELECTION Model and Capacity □ B0201-105-V02 □ B0201-105P-V02 □ B0201-205-V02 □ B0201-205P-V02 Electric Supply □ 220 V □ 50 Hz □ 60 Hz Vacuum Pump - Rate □ 15 m3/h □ 35 m3/h Accessories □ Wire baskets □ Ground support for baskets □ Wall support for baskets Supplies □ Sterilant 150 mL □ Packaging □ Autoclave tapes □ Incubator □ Biological indicator □ Chemical indicator □ Sealer Electric Tension Stabilizer □ Command only □ Online - The complete equipment Startup Kit □ With kit □ Without kit Services □ Validation and qualification □ Corrective maintenance □ Preventive maintenance □ Spare part kit APPLICATION Low temperature sterilizer, for processing materials sensitive to heat and humidity, using Hydrogen Peroxide steam and plasma. It allows quick return to use of metallic and non-metallic medical devices, with or without lumen, including those manufactured in heat-and humidity-sensitive materials, optical, glassware and others without leaving residues and without damaging the processed materials. MODEL AND CAPACITY B0201-105-V02 - 108L with single door. B0201-105P-V02 - 108L with double door. B0201-205-V02 - 234L with single door. B0201-205P-V02 - 234L with double door. MAIN FEATURES With modern and ergonomic design, tailored to the average standards of the user population, with reduced size and weight, easy to transport and move through hallways, doors and elevators. Easy installation, needing only electrical connection to grounded outlet, single-phase 220 V. No additional inputs such as water, steam, compressed air, exhaust and drainage. The hydrogen peroxide used in the process, is transformed into oxygen and water steam at the end of the process. Ease of transport given its reduced dimensions and low weight, does not require structural reinforcement at the installation site. Silent and economical operation at low power consumption, minimal heat radiation, does not add heat load to the air conditioning system. Ingredient in plastic bottles with 150 mL of H2O2 at a concentration of 50%, with RFID tag, prevents the use of peroxide with expired validity, or bottle reuse. Each flask can generate from 7 up to 15 cycles, according to the selected profile. Cycles carried out at temperatures of 50o C and in deep vacuum level. Low sensitivity to humidity. The control instrumentation identifies the presence of humidity in load and performs a pre-drying procedure, capable of preventing abortion of cycles with slightly humid loads. Sterilization by hydrogen peroxide is not usable for liquid loads, powders, tissues and cellulose-containing materials. STANDARDS Designs, materials and construction of the equipment meet the specifications of standards and directives listed below: • ANSI/AAMI/ISO 14937:2014 - Sterilization of health care products - General requirements for characterization of a sterilizing agent and the development, validation and routine control of a sterilization process for medical devices; • NBR ISO 14971:2009 - Products for health -Risk Management Application of health products; • IEC 61010-1:2013 - Safety requirements for electrical equipment for measurement, control and laboratory use - Part 1: General requirements; • IEC 61010-2-040:2005 - Safety requirements for electrical equipment for measurement, control and laboratory use - Part 2 - 040: Particular requirements for sterilizers and washer-disinfectors used to treat medical materials; • IEC 61326-1:2012 - Electrical equipment for measurement, control and laboratory use - EMC requirements - Part 1: General requirements; Essentials requirement medical device directive 93/42 EEC; • ANSI/AAMI/IEC 62304:2006 - Medical device software - Software life cycle processes; • AN INTERNATIONAL CODE ASME Boiler & Pressure Vessel Code - Section II - Part D -Properties (Customary); • AN INTERNATIONAL CODE ASME: Boiler & Pressure Vessel Code - Section VIII - Division I - Rules for Construction of Pressure Vessels; • NBR 5601:1998 - Stainless Steels; • NBR 5410:2004 - Low voltage electrical installations; • NBR 5419:2005 - Protection of structures against lightning. REGULATORY PARTS • RDC N° 56:2001 -Minimum requirements to prove the safety and efficacy of health products.
Open the catalog to page 1
Hydrogen Peroxide Sterilizer - PHB COMMAND, CONTROL AND INSTRUMENTATION Command: dedicated micro-processed controller, PLC industrial type, with routine self-tests, selfdiagnosis and supervision, controls the dosage of sterilant at the beginning of the cycle and allows the monitoring the process steps, the range of programmed parameters - time, temperature and pressure - and failures through direct reading on the digital display type IHM, equipped with a Touch screen 10” display. Graphic interface: designed and studied to ensure cognitive ergonomics, usability, fluidity and ease of...
Open the catalog to page 2
Hydrogen Peroxide Sterilizer - PHB SUPPLIES AND ACCESSORIES • D0104-003: Baskets Constructed in stainless steel AISI 304 (optionally AISI 316) for the storage of packages at the material center, operating room and others. • M0600-150-001: 50% H,O, solution Sterilant for exclusive use in BAUMER sterilizers - aqueous solution containing 50% hydrogen peroxide. Presentation: sealed bottles of 150 mL, with RFID tag, in packs of six bottles. • M0101-001-H: Marking tape With lists printed with ink sensitive to the presence of hydrogen peroxide in the tape surface. Presentation: Roll of 50 m and a...
Open the catalog to page 3
Minimum Distance Hydrogen Peroxide Sterilizer - PHB FRAME WITH PLANT SIGHT AND EQUIPMENT PERSPECTIVE Note: One-door sterilizer:there should be a minimum distance of 0.05 m between the bottom and the wall.
Open the catalog to page 4
BAUMER Hydrogen Peroxide Sterilizer - PHB INPUTS INTENDED FOR PRODUCT OPERATION The Hydrogen Peroxide Sterilizers - PHB Baumer have inputs for process preparation and control, as presented below. s a I Av. Arnolfo de Azevedo, 210 • Pacaembu • Sao Paulo/ SP • 01236-030 • PO Box: 1081^Phone: 11 3670 0000 • Fax: 11 3670 0053 aumer . . | www.baumer.com.br • e-mail: cmlbh@baumer.com.br
Open the catalog to page 5All BAUMER catalogs and technical brochures
-
Prostheses
36 Pages
-
LED SURGICAL FOCUS - QUANTA
2 Pages
-
Conquest Vertex
4 Pages
-
Steam Sterilizer HI VAC II
6 Pages
-
Trolleys and Racks
2 Pages
-
Drying cabinet EA-34-03
1 Pages
-
Ultrasonic Reprocessor
5 Pages
-
BIPOLAR
3 Pages
-
Quanta
2 Pages
-
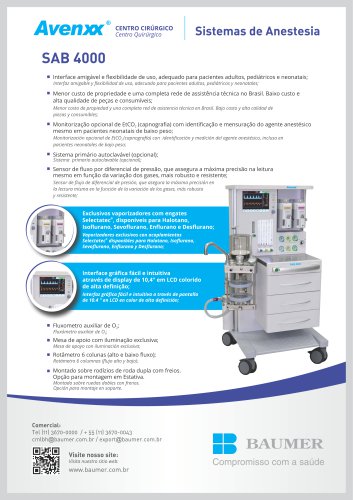
SAB 4000
2 Pages
-
C ONTROLE DE INFEC ÇÃ O
24 Pages
-
SPEED II
6 Pages
-
VEGA
8 Pages
-
Vega MAXX
4 Pages
-
CYGNUS
2 Pages