
Catalog excerpts

Staple Line Reinforcement Beijing Biosis Healing Biological Technology Co.,Ltd
Open the catalog to page 1
About us Company profile Founded in 2012, Biosis Healing is a well-know brand biological medical company in China, committed to providing innovative and comprehensive SIS biological materials for the whole surgery for the tissue repair and regeneration. Based on the SIS core technology, a series of implantable SIS material for tissue repair have been developed, covering a number of important surgical fields such as cardiac surgery, vascular surgery, gastrointestinal surgery, abdominal surgery, anorectal surgery, thoracic surgery, neurosurgery, orthopedics, oral and maxillofacial surgery...
Open the catalog to page 3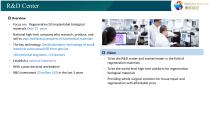
R&D Center R&D Center p Overview ‐ Focus on:Regenerative SIS Implantable biological materials Over 15 years ‐ National high-tech company who research, produce, and sell its own intellectual property of biomedical materials ‐ The key technology: Decellularization technology of small intestinal submucosa(SIS) from porcine ‐ >60 technical engineers, >10 doctors ‐ Establish a national laboratory ‐ With a post-doctoral workstation ‐ R&D investment 20 million USD in the last 3 years p Vision ‐ To be the R&D center and market leader in the field of regeneration materials ‐ To be the world-level...
Open the catalog to page 4
Company Milestone Company Milestone SIS hernia graft has got CE in 2021; Biological Anal fistula plug and Dura mesh has got CFDA in 2021 SIS hernia Patch got CFDA priority approval in 2018; Obtained Beijing S&T process progress Award National High-Tech Enterprise in 2016 Obtained a fund for innovation projects as a technology-based enterprise Zhongguancun high-tech enterprise in 2013 Dental membrane and stapleline reinforcement got CFDA in 2022 5 surgical staplers have got FDA (510K) Wu Jieping Medical Foundation--Biosis healing Excellent Surgery Special Fund Project in 2019 77 authorized...
Open the catalog to page 5
Inventional patents Inventional patents p 103 authorized national inventional patents p 21 authorized international inventional patents
Open the catalog to page 6
Product Ranges General Surgery Thoracic Surgery Vascular surgery Cardiology Oral Cavity Otology Neurosurgery Gynecology Orthopedics Stomatology Wound care Urology etc.
Open the catalog to page 7
What’s SIS Material SIS tissue-repair mesh is derived from the porcine small intestinal submucosa (SIS), composed of an extracelluar matrix (ECM), widely used in soft tissue repair for the whole body . p Feature ‐ Completely degradable: 1-6 months depends on different layers ‐ Storage at room temperature: temperature 10-30; humidity: ≤80% ‐ Process type: dried, non-crosslinked ‐ Sterilization method: ethylene oxide (EO) p Indications ‐ Used for implantation and reinforcement of weak soft tissues, including ventural hernia , inguinal hernia, vascular repair, dural repair, staple line...
Open the catalog to page 9
Tissue regeneration process p Regenerative repair is the trend to replace traditional filling implant materials ‐ Regenerative repair VS replacement repair ‐ Complete degradation VS incomplete degradation ‐ Healthy tissue repair > scar tissue repair p The “soil” and “signal” of tissue regeneration • Removal of immunogenic (cell, DNA, a-GAL antigen), good histocompatibility Retain natural extracellular matrix (ECM) and biological signals to induce tissue regeneration and repair ECM Promote tissue growth Tissue Regeneration Tissue regeneration Cell proliferation & differentiation Seed Cells
Open the catalog to page 10
ü Degrading while regenerating Good anti-adhesion properties Excellent antibacterial properti
Open the catalog to page 11
SIS Material Components PUBMED中SIS发表论文数 Chondroitin/Hyaluronic Acid Growth factor Type I/III Porcine Small intestinal submucosa material(SIS)
Open the catalog to page 12
SIS material introduction ( tensile strength testing ) ü Excellent tensile strength and good adhesion of Can be sutured continuously without tearing off the seam Easy to expand, effectively prevent air leakage and liquid leakage Seal the possible breakage and pinhole leakage No contracture, will not cause anastomotic stenosis Tensile limit rate
Open the catalog to page 13
SIS material introduction ( biological activity testing ) Reliable mechanical properties Relative Abundance Growth factor testing Relative Abundance Mass spectrometry testing for bioactive substances FGF-2 in SIS material Results: The basic growth factor in SIS material was detected by ELISA method. It was found that its FGF-2 content was over 43% in the original tissue, which proved that it has induced regeneration effect on tissues.
Open the catalog to page 14
SIS material introduction ( Immunogens removal ) DNA Residual ng/mg From HE stained sections, no residual cells The SIS material is immunogenicity removal (removing immunogens such as cells, DNA, and a-GAL etc, and the DNA residual is lowest of 3.8 ng/mg ) to achieve good histocompatibility. 脱细胞后 After Content testing of the α-Gal for the VIDASIS before and after
Open the catalog to page 15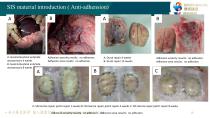
SIS material introduction ( Anti-adhension) B A: Gastrointestinal end/side anastomosis 4 weeks B: Gastrointestinal end/side anastomosis 8 weeks Adhesion severity results: no adhesion; Adhesion area results: no adhesion. A: Dural repair 4 weeks B: Dural repair 12 weeks Adhesion severity results: no adhesion; Adhesion area results: no adhesion. A: SIS Hernia repair patch repair 2 weeks B: SIS Hernia repair patch repair 4 weeks C: SIS Hernia repair patch repair 8 weeks Adhesion severity results: no adhesion; Adhesion area results: no adhesion.
Open the catalog to page 16
Advance Functional Materials Advanced Healthcare Materials Composite Part B Cell Proliferation Materialstoday BIO Regenerative Biomaterials
Open the catalog to page 17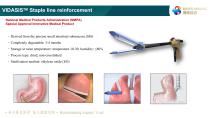
VIDASISTM Staple line reinforcement National Medical Products Administration (NMPA) Special Approval Innovative Medical Product ‐ Derived from the porcine small intestinal submucosa (SIS) ‐ Completely degradable: 3-4 months ‐ Storage at room temperature: temperature 10-30; humidity: ≤80% ‐ Process type: dried, non-crosslinked ‐ Sterilization method: ethylene o
Open the catalog to page 19
VIDASISTM Staple line reinforcement Why is the anastomotic bleeding ? ①Uneven thickness of cut tissue: When the tissue is too thick, the compression stress of the titanium staple is excessively concentrated, and the tissue is torn and bleeding; The tissue is too thin, and part of the tissue is not compressed tightly, resulting in cross-sectional bleeding. ②Incomplete hemostasis at the tissue stump during the operation, hematoma formed after anastomosis, postoperative anastomotic leakage rupture and bleeding ③Bowel wall edema, mucosal tear during suturing ④The anastomotic staple happened to...
Open the catalog to page 20All Beijing Biosis Healing Biological Technology catalogs and technical brochures
-
Biosis vision screener FD16
11 Pages
-
Biosis retinal camera R9000
11 Pages

















