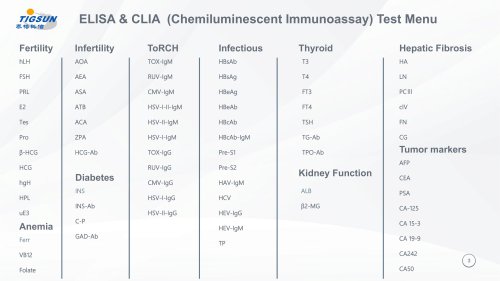
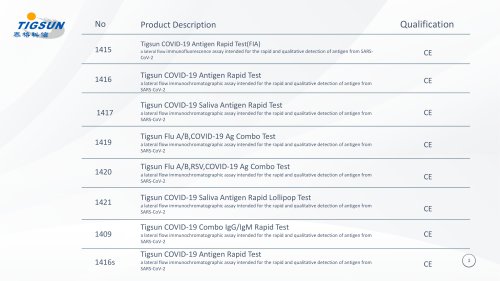
Catalog excerpts

Tigsun COVID-19 Antigen Rapid Test Clinical Validation Report Product name: Tigsun COVID-19 Antigen Rapid Test Model & specification: 1 test/kit, 1 Individual package Type of clinical trial: Clinical validation Start date of clinical trial: Nov. 2, 2020 Completion date of clinical trial: Mar. 3, 2021 Beijing Tigsun Diagnostics Co., Ltd.
Open the catalog to page 1
Abstract To evaluate the Tigsun COVID-19 Antigen Rapid Test (the “Tigsun Kit” for short) produced by Beijing Tigsun Diagnostics Co.,Ltd. (“the Company” for short) for clinical application in qualitative detection of the content of SARS-CoV-2 antigen in clinical samples (nasal swab samples), The medical institutions conducted a clinical study on the test cassette contained therein. A total of 516 nasal swab samples were collected in this clinical trial, including 190 positive samples and 326 negative samples confirmed by the COVID-19 diagnosis and treatment protocol. The Novel Coronavirus...
Open the catalog to page 2
1. Introduction Since December 2019, continuous surveillance on influenza and relevant diseases had been carried out, and several patients with viral pneumonia had been found and diagnosed with viral pneumonia/pulmonary infection. Relevant viruses were typed for detection. On January 7, 2020, the laboratory detected a novel coronavirus. The “2019 novel coronavirus (2019-nCoV) ” was identified in the viral pneumonia cases and named by the World Health Organization (WHO) on January 12, 2020. The 2019nCoV infected cases typically have symptoms like fever, fatigue, dry cough as the main...
Open the catalog to page 3
one collected for PCR detection and one collected for antigen test. Samples for PCR test are Blinded before testing, and unblinded after all test are finished. After the specialist have collected the nasal sample, 516 patients were chosen in all. 3.2 Trial design and study method selection 3.2.1 Sample size and sample size determination basis To ensure that the results are statistically significant, sufficient positive samples should be covered in this evaluation, such as no less than 150 positive samples of nasal swabs. 3.2.2 Sample selection criteria, inclusion criteria, exclusion...
Open the catalog to page 4
3 ) The diagnostic information of the sample is found to be missing or untraceable before statistics. 4 ) Samples with incomplete information 3.3 The determination of the comparative method In order to fully evaluate the clinical performance of this product, NAT and clinical diagnosis results were used as the control. Product information used in clinical trials: 3.4 Clinical evaluation method The reagents and clinical results are mainly represented in a four-grid table (as shown in Table 1). The table is self-explanatory, that is, it has table titles, table notes and number of cases SPSS...
Open the catalog to page 5
as well as Sensitivity Ct <32 and Sensitivity Ct <30 of the Test Kit and nucleic acid detection results, and summarize the indicators in the form of four-fold table. The results are as follows: Table 1 Summary of the Test Kit and Nucleic Acid Detection Results
Open the catalog to page 6
Table 5 Data Analysis It can be seen from Table 1 that among the 190 samples in the positive group, 186 cases are positive and 4 cases are negative. Among the 326 samples in the negative group, 324 cases are negative and 2 cases are positive. The diagnostic specificity, diagnostic sensitivity and total coincidence rate are all over 95%, Sensitivity Ct <32 is 98.92%, and Sensitivity Ct <30 is 100.00%, which indicates that the Test Kit has good diagnostic sensitivity and specificity in clinical performance, and is in good consistency with the Reference Kit. 5. Discussion and Conclusions A...
Open the catalog to page 7All Beijing Tigsun Diagnostics Co catalogs and technical brochures
-
POCT prodcuts catalog
2 Pages
-
Elisa Product catalog
1 Pages