Catalog excerpts

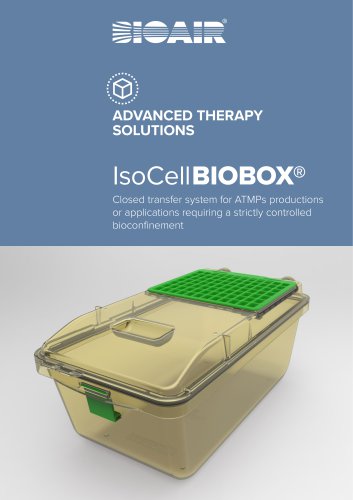
ADVANCED THERAPY SOLUTIONS Closed transfer system for ATMPs productions or applications requiring a strictly controlled bioconfinement
Open the catalog to page 1
The concept of bioconfinement A new frontier in Regenerative Medicine and in Advanced Therapy Medicinal Products (ATMPs) is represented by the growing use of Isolators, also defined “closed systems”, instead of using classical cleanrooms (defined as “open systems”). The use of these types of systems has become strongly recommended for the production of ATMP as established by the regulatory framework proposed by the Committee for Advanced Therapies (CAT). The purpose of using Isolators is to set up a bio-confined optimized working environment aiming at reducing production risks, management...
Open the catalog to page 2
How to use the IsoCell BIOBOX® in aspetic mode Introduction in the isolator of an empty, sterile IsocellBIOBOX and sterile loading procedure. From Grade D room to work area Grade A, loading and transfer to external incubator 1. Empty IsocellBIOBOX in the transfer hatch: closing transfer hatch external port; decontamination of the external surface of IsocellBIOBOX; 2. Achievement of Grade A; 3. IsocellBIOBOX lid opening; 4. Open empty IsocellBIOBOX: communication port opening between work area and transfer hatch; 5. Automatic introduction of the tray in the work area with the open empty...
Open the catalog to page 3
Workflow in open system with IsoCell BIOBOX® OPEN HOOD Grade A Obviously IsoCell IOBOX® B can easily been used in open systems as well, for transfer of samples inside the Grade B Clean Room or outside of it (figure 3). WORKING AREA EXTERNAL ENVIRONMENT ROOM Grade B figure 3 To date, apart from some patented commercial solutions with a high economic impact, IsoCell IOBOX® represents an unique, reliable, B GMP compliant, low-cost, easy-to-use system that guarantees the bio-confinement in Grade A during the necessary transfers of semi- or finished products when these products are moved...
Open the catalog to page 4
IsocellBIOBOX® tests Some experiments performed on two different cell lines like bone marrow mesenchimal stem cells (MSC-BM) and HACAT cells (transformed aneuploid immortal keratinocyte cell line from adult human skin) demonstrate that IsoCell IOBOX® use does not adversely affect the B maintenance and viability of the cells within it. Furthermore, the H14 filter membrane of IsoCell IOBOX® do not influence gas exchange between B cells and environment and avoid any possible contamination to penetrate inside the IsoCell IOBOX® when hermetically locked. B Test 1/ Proliferation No significant...
Open the catalog to page 5
IsoCell IOBOX® B IsoCell IOBOX® is a closed transfer system made in a special moulded B plastic material (PSU or PPSU) resistant to thermal and/or chemicals sterilization cycles (H2O2) equipped with an HEPA filter membrane that allows gas exchange (CO2 and O2) but avoid the possibility of external particulate contamination to enter the airtight container. maximum temperature: 134 c (273° ) for ° f 5-10 min. maximum inside and outside the IsocellBIOBOX® REPLACEABLE HEPA FILTER that allows gaz exchange with controlled condition for CO2, O2, humidity and prevent external contamination...
Open the catalog to page 6All BioAir catalogs and technical brochures
-
BIOAIR ISOLATION TECHNOLOGY
6 Pages
-
S@fegrow 188
3 Pages
-
S@fe3 Class III MICROBIOLOG
3 Pages
-
SAFEMATE TOTAL
3 Pages
-
Safemate Cyto
5 Pages
-
AURA PCRTM
3 Pages
-
AURA Mini Series
3 Pages
-
AURA VERICAL SDV Series
3 Pages
-
AURA HZ Series
3 Pages
-
SAFEGROW PRO CO2 INCUBATORS
12 Pages
-
YOUR SAFETY IS OUR COMMITMENT
28 Pages