
Catalog excerpts
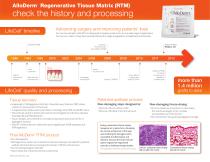
AlloDerm Regenerative Tissue Matrix (RTM) ® check the history and processing Advancing surgery and improving patients’ lives LifeCell™ timeline For over two decades, LifeCell™ has developed innovative products for use in a wide range of applications. Our success could not have been possible without the insight and guidance of healthcare professionals. burn treatment LifeCell Corporation formed Strattice™ Reconstructive Tissue Matrix (RTM) head and neck reconstruction breast reconstruction challenging hernia repair AlloDerm® RTM for breast reconstruction available in bi-lateral pairs breast plastic surgery with Strattice™ RTM LifeCell™ quality and processing more than 1.4 million grafts to date Tissue recovery Patented acellular process • Independent, FDA Registered 3rd Party Tissue Recovery Partners (TRP) obtain consent for procurement of the tissue • Comprehensive medical and social history screening meets FDA and AATB criteria • Donor blood sample is tested and must be found negative for infectious diseases including HIV and Hepatitis B & C • Tissue samples are screened for microbial contaminants and must be free of pathogenic bacteria • LifeCell™ medical directors determine donor eligibility per AATB standards and FDA regulations Non-damaging steps designed to: Non-damaging freeze-drying: • Remove cells on surface of skin • Remove cells deep in the dermal tissue • Process creates an amorphous ice that retains the structural integrity of the complex microarchitecture of the dermis1 • Non-destructive drying and packaging for storage Final AlloDerm RTM product ® • No cells detected • Although donor tissues are screened for infectious diseases, the LifeCell™ patented acellular dermal matrix processing demonstrates >99.9% viral reduction • No microbial pathogens detected • Intact matrix and critical biochemical components SPMP12289 REV A DEC 2012 During conventional freeze-drying, hexagonal ice crystals form, disrupting the normal architecture of this layer and rendering the damaged matrix susceptible to inflammation and rejection, because the hosts immune system regards the fragmented subunits as individual foreign bodies.1 40x magnification, Verhoeff-van Geison (stain for elastin) LifeCell™ patented freeze-drying did not alter the m
Open the catalog to page 1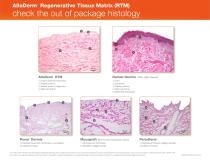
AlloDerm Regenerative Tissue Matrix (RTM) ® check the out of package histology 1 Human Dermis Intact basement membrane Papillary dermis Basket weave configuration Reticular dermis Cells Epidermis Papillary dermis Reticular dermis Basement membrane Puros Dermis 1) Modified basement membrane, no undulation 2) Cellular remnants 1) No basement membrane 2) Modified collagen bundle (Non-human extracellular matrix) 1) Separation between collagen bundles 2) Cellular remnants 1) LH Holton, D Kim, RP Silverman, ED Rodriguez, N Singh, NH Goldberg. Human acellular dermal matrix for repair of abdominal...
Open the catalog to page 2All BioHorizons catalogs and technical brochures
-
International Product Catalog
12 Pages
-
Biologics Catalog
28 Pages
-
External Dental Implant Catalog
44 Pages
-
patientconnect
24 Pages
-
prosthetics catalog 2017
36 Pages
-
External Dental Implant
44 Pages
-
Surgical Motors
2 Pages
-
laser-lok
48 Pages
-
biologics product catalog
12 Pages
-
one-piece 3.0 & overdenture
28 Pages
-
laser-lok 3.0
20 Pages
-
Hu-Friedy® dental instruments
24 Pages
-
guided surgery kit
24 Pages
-
tapered internal implant system
28 Pages
Archived catalogs
-
prosthetics catalog
40 Pages























