 Website:
Bonegraft Biomaterials
Website:
Bonegraft Biomaterials
Catalog excerpts

About Powerbone Mission and Vision Pioneer of R&D Powerbone Certificates Powerbone Production Standards Product References and Sizes
Open the catalog to page 2
Triabone R&D activites take place at Ege University Technology Development Centre under ISO-7 (Class 10000). Since 2016 production to ISO-5, ISO 6 and ISO-7 (Class 100,1000,10,000) conditions takes place in Manisa. A biotechnology company that focuses on the development, manufacturing and marketing of innovative and functional biomaterials. Powerbone Biomaterials Co. is one of the leading company in the world that can produce osteoinductive and osteoconductive synthetic bone graft materials, barrier membrane, bone cement and cartilage graft. In addition to expert employees, high technology...
Open the catalog to page 3
Our Vision; Our aims are to become a globally competitive and respected biomaterials company that draws attention to developed strategic innovations and to bring the name and power of our country in the field of biomaterials manufacturing to the top. Our Mission; To have the widest and most innovative biomaterials range in the field of orthopedic, spine, trauma, and dental surgeries with the maximum investment power to R&D studies.
Open the catalog to page 4
R&D-Oriented Production Production All raw materials and equipment used in production processes are supplied from European and American companies with the highest technological standards. Powerbone’s modern production facility is electronically controlled. Multiple production parameters are observed in real time 24/7 according to ISO7 standard. The production areas ar monitored according to ISO14644 Class 10.000 and Fed.Std.209 D, ISO5. ISO 14644 is applied to the clean rooms and packaging areas to Fed.Std.209D TSE approved TS EN ISO 13485 Quality Management System Our products bear the CE...
Open the catalog to page 5
Quality control is carried out in accordance with production instructions using measurement devices unique to each specific medical device. Our R&D unit, located in Ege University Technology Development Zone has a special focus on novel synthetic technologies designed for tissue engineering and regeneration of bone and cartilage tissue. BIOMATERIAL INNOVATION REPLACED BY NEW BONE • Innovative Flexible Bone Graft • Connected high pore structure, cross-section of bone grafts for different geometries and sizes • Bone graft in injectable form that does not require mixing
Open the catalog to page 6
•CE Certificates for each product groups
Open the catalog to page 7
SILICATE ADDITIVE GRANULE, STICK & BLOCK & WEDGE Safe, Biocompatible and Sterile Calcium phosphate provides a conductive scaffold for blood vessels and bone stimulating cells - a structure very similar to the mineral component of natural bone. Powerbone grafts are supplied sterile and CE marked as a Class III Medical Device according to 93/42 / EC directive. Biocompatibility (in vitro and in vivo), biodegradation, bioburden and sterility tests are applied to each material. The high porosity of Powerbone products support initial clot stabilisation. Powerbone materials include silicate which...
Open the catalog to page 9
FLEXIBLE STRIP / GRAFT Powerbone Flexible Strip Implantation; Powerbone Flexible Strip is a biodegradable synthetic graft that is easy to use due to its high elasticity, especially in bone/tissue defects of the orthopedic pelvis and lower extremities as well as posteriolateral spinal fusion cases. Powerbone Flexible Strip can be applied to the surgical area directly or combined with bone marrow aspirate or blood. Powerbone Flexible Strip consists of silicate-added B-TCP embedded inside a PLA based synthetic polymer lattice of varying thicknesses. Osteoinductive characteristic are achieved...
Open the catalog to page 10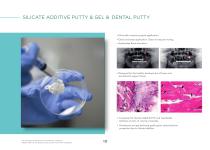
SILICATE ADDITIVE PUTTY & GEL & DENTAL PUTTY • Minimally invasive surgical application • Quick and easy application. Does not require mixing. • Accelerates Bone formation. • Designed for the healthy development of bone and periodontal support tissue. • Composed of silicate added B-TCP and resorbable cellulose carriers of varying viscosities. • Powerbone syringe delivered grafts gains osteoinductive properties due to silicate addition. For product dimensions and reference numbers, please refer to the product lists at the end of the catal
Open the catalog to page 11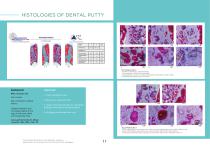
HISTOLOGIES OF DENTAL PUTTY Fig. 2 Details Section A a-f) PDP surrounded by connective tissue. b) Occasionally, Osteoid (O) detectable. d-f) Extraordinary amount of multinucleated giant cells (MNGs, Asterisk*) visible. d-f) PDP appears biphasic (grey and pink) Background Male, 25 years old. CASE STUDY 1. Initial radiological view. Non-smoker. Non-contr�butory med�cal h�story. Surg�cal extract�on and �mmed�ate graft�ng of the large 4-wall bone defect w�th Powerbone Putty. 2. Removal of impacted tooth. 3. Usage of Powerbone Putty for immediate grafting of the large 4-wall bone defect. 4....
Open the catalog to page 12
DENTAL BARRIER MEMBRANE Powerbone Barrier Membrane is: -Designed for the healthy development of bone and periodontal support tissue. -Composed of a poly (lactic acid) based synthetic polymer that is biocompatible and resorbable with an excellent safety profile in medical applications. The three-layered structure of Powerbone Barrier Membrane prevents the migration of epithelial and fibroblast cells, selectively supporting the healthy development of bone and periodental tissues. Powerbone Barrier Membrane preserves its structure for 10-12 weeks and is completely absorb. Powerbone Barrier...
Open the catalog to page 13
CHONDRO MATRIX Chondro Matrix low (a) and high (b Powerbone Chondro Matrix is one-step, hydrophilic, sterile, bioresorbable, CE marked, cell-free implant used to treat articular cartilage defects in the knee, ankle or hip. It uses the biological potential of stem cells to restore damaged cartilage tissue in the joints. Use of Powerbone Chondro Matrix; 1 • Powerbone Chondro Matrix is composed of biomedical grade Polylactic acid (PLA) to provide structural support for 1-2 months and sodium hyaluronate (hyaluronic acid) to promote chondrogenesis. Microfracturing Mesenchymal stem cells are...
Open the catalog to page 14
