
Catalog excerpts

KIT ENDORET® ALVEOLUS POST-EXTRACTION
Open the catalog to page 1
KIT FOR ALVEOLUSES POST-EXTRACTION ENDORET® (PRGF®) TECHNOLOGY, SHORTER PREPARATION TIME BTI Biotechnology Institute presents the new Kit KMU16, specifically designed for the treatment of the postextraction alveolus that consists of material for 15 clinical uses with the advantage that it makes the clinical times more profitable. · FAST WITHOUT THE NEED FOR ANTICOAGULANT NOR ACTIVATOR. · SIMPLE THE SAME PRODUCT OBTAINED, IN FEWER STEPS. · BIOLOGICAL THE FORMATION OF THE FIBRIN MEMBRANE AND THE CLOT IS FASTER, WHILE STILL PRESERVING THE RETENTION CAPACITY OF GROWTH FACTOR
Open the catalog to page 2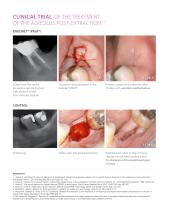
CLINICAL TRIAL OF THE TREATMENT OF THE ALVEOLUS POST-EXTRACTION (1) ENDORET® (PRGF®) 1ST DAY Initial X-ray. The molar presents a vertical fracture with absence of the inter-radicular septum. Extraction and placement of the Endoret®(PRGF®). 15 DAYS Primary closure of the alveolus after 15 days with excellent epithelisation. Defect after the dental extraction. 15 DAYS Epithelisation after 15 days Primary closing has not been achieved and the thickness of the keratinised gum is lower. BIOGRAPHY 1. Anitua E, Alkhraisat M, Orive G, Murias A. A randomized clinical trial evaluating plasma rich in...
Open the catalog to page 3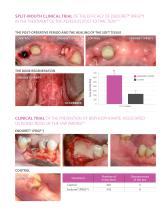
THE BONE REGENERATION ENDORET®(PRGF®) CLINICAL TRIAL OF THE PREVENTION OF BISPHOSPHONATE-ASSOCIATED OSTEONECROSIS OF THE JAW (BRONJ)(5) Treatment
Open the catalog to page 4
COMPONENTS THE NEW KMU16 KIT EXTRACTION SYSTEM FRACTIONING TUBES IDENTIFICATION LABELS PLASMA TRANSFER DEVICE (PTD2) EXTRACTION TUBES NO ADDITIVES Due to the lack of anticoagulant, the coagulation process is completely natural and much quicker, as it does not depend on the addition of activator. This kit contains material for 15 uses. BIOLOGICAL TREATMENT FOR THE POST-EXTRACTION ALVEOLUS 1. Improved healing in the soft tissue. Passive closure at 15 Days and the thickness of keratinised gum is double that of cases without Endoret®. 2. Improved post-operative experience for the Fibrin...
Open the catalog to page 5
BTI Comercial San Antonio, 15 • 5° 01005 Vitoria-Gasteiz (Alava) ■ SPAIN Tel: +34 945 140 024 Fax: +34 945 135 203 pedidos@bticomercial.com Subsidiaries USA 1730 Walton Road Suite 110 Blue Bell. PA 19422-1802 ■ USA Tel: (1) 215 646 4067 Fax: (1) 215 646 4066 info@bti-implant.us UK 870 The Crescent Colchester Business Park • Colchester Essex CO49YQ ■ Regno Unito Tel: (44) 01206580160 Fax: (44) 01206580161 info@bti-implant.co.uk GERMANY Mannheimer Str. 17 75179 Pforzheim • Deutschland Tel. +49 (0) 7231 428060 Fax +49 (0) 7231 4280615 info@bti-implant.de B.T.I. Biotechnology Institute S.L....
Open the catalog to page 6All BTI Biotechnology Institute catalogs and technical brochures
-
BTI Root Extractor
4 Pages
-
BTI UniCca® Surface
5 Pages
-
BTI Surgical Instruments
48 Pages
-
BTI Front Cutting Drill
6 Pages
-
KEXIM Implant Extraction Kit
4 Pages
-
ABUTMENT MULTI-IM
6 Pages
Archived catalogs
-
CAD/CAM DISC Blank
2 Pages



















