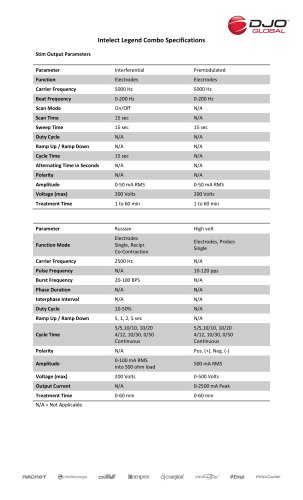
Website:
Chattanooga USA
Website:
Chattanooga USA
Group: Djo Global
Catalog excerpts
VitalStim® Plus Four Channel Electrotherapy System User Manual Operator and Installation Instructions
Open the catalog to page 1
VitalStim® Plus Electrotherapy System TABLE OF CONTENTS INTRODUCTION . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 FORWARD . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2 PRECAUTIONARY INSTRUCTIONS . . . . . . . . . . . . . . . . . . . . 2 GENERAL TERMINOLOGY . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3 SYSTEM SOFTWARE SYMBOLS . . . . . . . . . . . . . . . . . . . . . . . 3 DEVICE MARKINGS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4 INDICATIONS FOR USE . . . . . . . . . . . . . . . . ....
Open the catalog to page 2
VitalStim® Plus Electrotherapy System PRECAUTIONARY INSTRUCTIONS This manual is intended for users of VitalStim® Plus Electrotherapy System. It contains general information on operation, precautionary practices, and maintenance. In order to maximize use, efficiency, and the life of the system, please read this manual thoroughly and become familiar with the controls, as well as the accessories before operating the system. The precautionary instructions found in this section and throughout this manual are indicated by specific symbols. Understand these symbols and their definitions before...
Open the catalog to page 3
VitalStim® Plus Electrotherapy System GENERAL TERMINOLOGY The following are definitions for the terminology used throughout this manual. Study these terms to become familiar with them for ease of system operation and control functionality of the VitalStim® Plus Electrotherapy System. SYSTEM SOFTWARE SYMBOLS Back Arrow/Previous Screen Forward Arrow/ Next Screen sEMG Increase/Decrease Parameter sEMG+VMS Electrode Placement Scroll Up or Down in a text box Modality Description Select Custom Protocols Patient Data Micro SD Card indicator Anatomical Library Hand switch indicator Battery voltage...
Open the catalog to page 4
VitalStim® Plus Electrotherapy System DESCRIPTION OF DEVICE MARKINGS The markings on the unit are assurance of its conformity to the highest applicable standards of medical equipment safety and electromagnetic compatibility. One or more of the following markings may appear on the device: Refer to Instructional Manual Booklet . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Neuromuscular Stimulation (STIM) and sEMG +Stimulation should not be used by Patients fitted with demand style cardiac pacemakers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . ....
Open the catalog to page 5
VitalStim® Plus Electrotherapy System ELECTROTHERAPY, sEMG+VMS INDICATIONS Indications The sEMG intended uses are surface electromyography biofeedback for relaxation training and muscle reeducation. For VMS™ - VitalStim Waveforms and sEMG Triggered Stimulation. • Muscle re-education by application of external stimulation to the muscles necessary for pharyngeal contraction. Intended Uses- VMS™ Waveform This device should not be used when cancerous lesions are present in the treatment area. VMS waveform is a square symmetrical biphasic waveform with the application for use on the musculature...
Open the catalog to page 6
VitalStim® Plus Electrotherapy System Additional Precautions • Caution should be used for patients with suspected or diagnosed epilepsy Caution should be used for patients with suspected or diagnosed heart problems Caution should be used in the presence of the following: - When there is a tendency to hemorrhage following acute trauma or fracture - Following recent surgical procedures when muscle contraction may disrupt the healing process - Over areas of the skin that lack normal sensation • Some patients may experience skin irritation or hypersensitivity due to the electrical stimulation...
Open the catalog to page 7
DEVICE DESCRIPTION VitalStim® Plus Electrotherapy System PRODUCT DESCRIPTION The VitalStim® Plus Electrotherapy System is a 2 Channel sEMG and 4 Channel electrotherapy system used in treating patients with oral-pharyngeal dysfunctions (dysphagia) and disorders of the head and neck, with Bluetooth connection to PC software. To maximize functionality and life of VitalStim® Plus Electrotherapy System, be sure to: • Stay current with the latest clinical developments in the field of electrotherapy, sEMG (Surface Electromyography), sEMG + Stim (Surface Electromyography with Triggered Stimulation)...
Open the catalog to page 8
DEVICE DESCRIPTION VitalStim® Plus Electrotherapy System OPERATOR INTERFACE Front Controls VitalStim® Plus Electrotherapy System Operator Interface contains all the functions and controls necessary for operator access to all operator utilities, modalities, and parameters for modification and system set up. 1. Color Display 2. BACK button 3. HOME button 4. Clinical Resource Library button 6. STOP button 7. START/PAUSE button 9. Ch3 Lead Wire Connector (STIM) 10. Ch4 Lead Wire Connector (STIM) 11. Operator Remote Switch Connector Front Panel and Battery Compartment 12. Ch2 Lead Wire Connector...
Open the catalog to page 9
GENERAL WARNINGS AND PRECAUTIONS VitalStim® Plus Electrotherapy System • Read, understand, and practice the precautionary and operating instructions. Know the limitations and hazards associated with using any electrical stimulation. Observe the precautionary and operational decals placed on the unit. • Inspect lead wires and associated connectors for signs of damage before each use. Replace damaged lead wires immediately with new before any treatment is applied. • Electrode placement and stimulation settings should be based on the guidance of the prescribing practitioner or other licensed...
Open the catalog to page 10
GENERAL WARNINGS AND PRECAUTIONS VitalStim® Plus Electrotherapy System WARNING • Powered muscle stimulators should be used only with the leads and electrodes recommended for use by the manufacturer. • Before administering any treatment to a patient you should become acquainted with the operating procedures for each mode of treatment available, as well as the indications, contraindications, warnings and precautions. Consult other resources for additional information regarding the application of each mode of treatment. • Simultaneous connection of a patient to high frequency surgical...
Open the catalog to page 11All Chattanooga USA catalogs and technical brochures
-
Montane Table LINE
33 Pages
-
CPM and Homecare
20 Pages
-
3D ActiveTrac™
4 Pages
-
CUBE Vet High Power Laser
2 Pages
-
CUBE Vet High Power Laser
8 Pages
-
CUBE High Power Laser
2 Pages
-
CUBE High Power Laser
8 Pages
-
Rehab/Theta/Physio
4 Pages
-
Wireless Professional
4 Pages
-
Mobile RPW Shockwave
4 Pages
-
VitalStim® Plus
2 Pages
-
VitalStim® Plus
4 Pages
-
Vectra® Neo THERAPY SYSTEM
1 Pages
-
Iontophoresis
8 Pages
-
Physical Medicine Modalities
28 Pages
-
Hot and Cold
12 Pages
-
Training and Mobility
12 Pages
-
Montane Tables
8 Pages
-
Treatment Tables
16 Pages
-
Intelect® Vet
8 Pages
-
VitalStim Therapy
8 Pages
-
Intelect® RPW Shockwave
16 Pages
-
PresSsion? Lymphedema Pump
4 Pages
-
Vectra GENISYS
8 Pages
-
Moveo XP
5 Pages
-
primera
2 Pages
-
Patient Lifts 2013
8 Pages
-
Adapta Summit 7
4 Pages
-
CPM and Homecare 2013
24 Pages
-
Physical Agent Modalities 2013
16 Pages
-
Intelecet SWD100 2009
6 Pages
Archived catalogs
-
Wireless Professional
8 Pages
-
AIRCAST PROCARE Product Catalog
139 Pages
-
Montane Tables Old
2 Pages
-
Moving Rehabilitation Forward
25 Pages
-
Rehabilitation Products Catalog
76 Pages
-
Vectra® Genisys
5 Pages