
Catalog excerpts

ORDERING INFORMATION THE MYNXGRIP'' Vascular Closure Device includes: (11 Balloon catheter with integrated sealant (1] 10ml locking syringe SIZE COLOR MYNX ORDER NUMBER To order the MYNXGRIP® Vascular Closure Device in the United States contact your local Cordis sales rep or customer service at 800.327.7714. To learn more visit cordis.com/mynx ONLY INDICATIONS FOR USE The MYNXGRIP' ' Device is indicated for use to seal femoral arterial and femoral venous access sites while reducing times to hemostasis and ambulation in patients who have undergone diagnostic or interventional endovascular procedures utilizing a 5F, 6F or 7F procedural sheath. PRECAUTIONS The MYNXGRIP® Device should only be used by a trained licensed physician or healthcare professional. The MYNXGRIP® Device should not be used in patients with a known allergy to PEG. WARNINGS Do not use if components or packaging appear to be damaged or defective or if any portion of the packaging has been previously opened. DO NOT REUSE OR RESTERILIZE. The MYNXGRIP'' Device is for single use only. The balloon catheter is loaded with a single hydrogel sealant. Reuse of the device would result in no delivery of hydrogel sealant. Do not use the MYNXGRIP'' Device if the puncture site is located above the most inferior border of the inferior epigastric artery (IEA] (for arterial application] and/ or above the inguinal ligament based upon osseus landmarks, since such a puncture site may result in a retroperitoneal hematoma/bleed. Perform a femoral angiogram or venogram to verify the location of the puncture site. Do not use the MYNXGRIP®' Device if the puncture is through the posterior wall or if there are multiple punctures, as such punctures may result in a retroperitoneal hematoma/bleed. Cordis. A Cardinal Health company Caution: Federal (USA] law restricts these devices to sale by or on the order of a physician. For information on indications, contraindications, warnings, precautions, and adverse events, see Full Instructions for Use. For Healthcare Professional Only. © 2016 Cardinal Health. All rights reserved. CORDIS, the Cordis LOGO, and MYNXGRIP are trademarks or registered trademarks of Cardinal Health. The MYNXGRIP®' Vascular Closure Device is manufactured by Cardinal Health and is part of the Cordis portfolio. MKT7783.E 11/16 MyNX MYNXGRIP*VASCULAR CLOSURE DEVICE STRONG Cordis. A Cardinal Health company
Open the catalog to page 1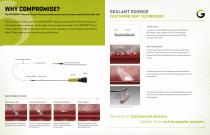
WHY COMPROMISE? The MYNXGRIP® Vascular Closure Device provides secure vascular closure without the trade-offs. SEALANT SCIENCE FEATURING GRIP TECHNOLOGY Built on the proven Mynx platform, the MYNXGRIP® Vascular Closure Device offers the security of mechanical closure combined with the safety of an extravascular sealant. The MYNXGRIP® Device offers a patient-friendly closure option with no sutures, clamping, or metal implants and dissolves within 30 days leaving nothing behind but a healed artery. The sealant in the MYNXGRIP® Device consists of Polyethylene Glycol (PEG), a water-soluble,...
Open the catalog to page 2All Cordis catalogs and technical brochures
-
STORQ Steerable Guidewire
4 Pages
-
Biopsy Forceps
4 Pages
Archived catalogs
-
Cordis PALMAZ Blue™
4 Pages
-
OPTEASE
12 Pages
-
Cordis PRECISEPRO RX
2 Pages
-
Steerable Guidewires
11 Pages
-
BIOPSY FORCEPS
4 Pages
-
Cordis STORQ
4 Pages
-
crossing portfolio
16 Pages
-
Cordis EMERALD™
2 Pages
-
SLEEK™ OTW
2 Pages
-
Endovascular catalog
123 Pages
-
VISTA BRITE TIP® Brochure
22 Pages
-
accessories-
6 Pages
-
diagnostic-catheters
112 Pages
-
Cordis BRITE TIP®
3 Pages
-
SABERX™
3 Pages
-
Cordis Carotid Systems
10 Pages
-
Cordis ExoSEal
12 Pages
-
Accessories
10 Pages
-
Biopsy Forceps Product Page
4 Pages
-
AquaTrack
3 Pages
-
Radial Solutions
38 Pages
-
Avanti®+
2 Pages
-
RadialSource
2 Pages
-
Diagnostic Solutions
23 Pages
-
ADROIT
7 Pages
-
PRECISE® PRO RX®
129 Pages
-
Product catalog
146 Pages












































