Website:
Depuy Synthes
Website:
Depuy Synthes
Group: Johnson & Johnson
Catalog excerpts

For the Attention of the Operating Surgeon: IMPORTANT INFORMATION ON THE DEPUY SYNTHES SYNFLATE SYSTEM DESCRIPTION The Synflate Vertebral Balloon is part of a modular system including an inflation system, a variety of access instruments, a biopsy kit, an access drill and implant options. All instruments and implants are available separately. This allows for maximum economy, flexibility, and precise surgical planning. Refer to the surgical technique guide for a full list of compatible instruments and implants. The Synflate Vertebral Balloon contains the following components: 1. Balloon-catheter 2. Stiffening wire 3. Syringe with Luer lock Once access is obtained, the Synflate Vertebral Balloon, attached to an inflation system, is used to reduce fractures and/or create a void in the cancellous bone of the vertebral body. The access instruments and inflation system are designed specifically for use with the Synflate Vertebral Balloon device. Alternate instrumentation should not be used with the Synflate Vertebral Balloon device. Refer to the corresponding Instructions for Use for the access instruments and inflation system for additional details regarding these devices. Additionally, adhere to the Instructions for Use for the specific PMMA based bone cement used during the procedure. The Synflate Vertebral Balloon, inflation system and access instruments are supplied sterile and packaged separately. The Synflate System has not been evaluated for safety and compatibility in the MR environment. The Synflate System has not been tested for heating or migration in the MR environment. Read the surgical technique guide carefully prior to use. To obtain a copy of the Synflate Surgical Technique Guide, contact your local DePuy Synthes sales representative. INDICATIONS The Synflate Vertebral Balloon System is intended to be used for the reduction of fractures and/or creation of a void in cancellous bone in the spine. This includes use during percutaneous vertebral augmentation. The system is to be used with cleared spinal polymethylmethacrylate (PMMA) bone cements indicated for use during percutaneous vertebral augmentation procedures, such as kyphoplasty. CONTRAINDICATIONS • Instability of posterior wall and/or pedicles • Infection • Severe bleeding • Known allergies to bone cement • Pregnancy • Fractures in which more than 75% of vertebral height is lost • Any known severe allergy to contrast material • When safe placement and inflation of the balloon is not possible due to vertebral dimensions or fracture pattern. WARNINGS The manufacturer is not responsible for any complications arising from incorrect diagnosis, choice of incorrect bone filler, incorrectly combined components and/or operating techniques, the limitations of treatment methods, or inadequate asepsis. This document is valid only on the date printed. If unsure of the print date, please re-print to ensure use of the latest revision of the IFU (available at www.e-ifu.com). The onus resides with the user to ensure that the most up-to-date IFU is
Open the catalog to page 1
It is the responsibility of the physician performing this procedure to determine the appropriateness of the product and specific techniques. − Do not leave the balloon implanted; the balloon material is not implant grade material. Breakage of the balloon or any part of the Synflate System (i.e. access instruments) may require intervention or retrieval. − Do not use air and/or other gas to inflate the balloon. Use only liquid contrast medium to inflate the balloon as indicated. − Do not inflate the balloon until it has been fully deployed in the vertebral body. Inflating the balloon prior to...
Open the catalog to page 2
For a transpedicular approach, if the pedicle is not large enough or stable enough to withstand the procedure, pedicle fracture may occur. When using bone cement, refer to the specific Instructions for Use for indications, contraindications, warnings, precautions, and adverse events related to bone cement. Inflation Chart (maximum recommended) 03.804.700S, Small Unconstrained Inflation Chart (unconstrained test conditions) 03.804.700S, Small Inflation Volume Inflation Pressure The device components should be stored in their original packaging and should not be removed from the protective...
Open the catalog to page 3All Depuy Synthes catalogs and technical brochures
-
ZERO-P NATURAL™PLATE
5 Pages
-
2.0 mm LCP® Distal Ulna Plate
20 Pages
-
2.4 mm VA LCP™
4 Pages
-
Building on Success
16 Pages
-
HEALIX ADVANCE
4 Pages
-
RADIUS OF CURVATURE
3 Pages
-
Introducing The Variable Angle
12 Pages
-
HEALIX Anchor™ 3.4 mm
2 Pages
-
Small Battery Drive II
4 Pages
-
HEALIX ADVANCE
4 Pages
-
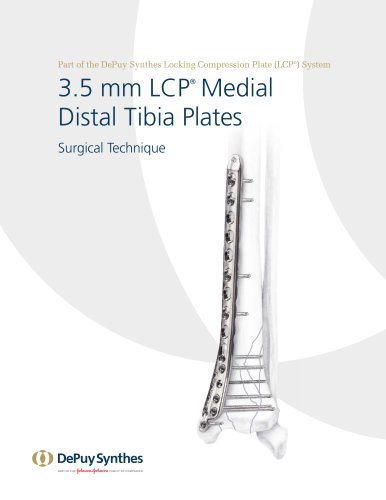
3.5 mm LCP™ Medial
15 Pages
-
Titanium Sternal Fixation System
34 Pages
-
MatrixRIB®FixationSystem
86 Pages
-
Mandible Trauma Solutions
2 Pages
-
Power line II
4 Pages
-
Concorde
28 Pages
-
LCP Intercarpal
31 Pages
-
LCS® COMPLETE™
2 Pages
-
Synthes TPLO.
8 Pages
-
SynFix-LR System
56 Pages
-
ATB Anterior Tension Band Plate
32 Pages
-
CONDUIT™
15 Pages
-
Brochure_FINAL
2 Pages
-
DePuy Synthes
81 Pages
-
Anspach
3 Pages
-
Orthopedic Foot Instruments
32 Pages
-
PINNACLE® Hip Solutions
12 Pages
-
Corail
24 Pages
-
S-ROM® NOILES™
68 Pages
-
TRI-LOCK® Product Rationale
12 Pages
-
Reclaim Surgical Technique
44 Pages
-
Speed
2 Pages
-
attune
80 Pages
-
HAMMERLOCK® 2
2 Pages
-
DePuy Glenoid Solutions
2 Pages
-
Trauma Solutions. Elbow
4 Pages
-
Polar
4 Pages
-
Alveolar Distractor.
4 Pages
-
Piezoelectric System
4 Pages
-
Air Power Line II
6 Pages
-
LCP Clavicle Hook Plate
4 Pages
-
TruMatch Pin Guides
16 Pages
-
P F N A
8 Pages
-
SKILL, DEDICATION,
16 Pages
-
Orthopaedics. Overview
20 Pages
-
DURALOC
16 Pages
-
Marathon Cemented Cup
20 Pages
-
REEF Surgical Technique
16 Pages
-
MatrixNEURO
8 Pages
-
Anspach XMax
4 Pages
-
Anspach eMax 2 Plus
4 Pages
-
Small Electric Drive
4 Pages
-
Air Pen Drive
4 Pages
-
Colibri II
4 Pages
-
Spine
25 Pages
-
Expert Hindfoot Arthrodesis Nail
48 Pages
-
LCP Distal Fibula Plates
32 Pages
-
TomoFix
60 Pages
-
Expert Tibial Nail PROtect
16 Pages
-
Expert Tibia Nail
84 Pages
-
Sacral Bars
16 Pages
-
Pelvic C-Clamp
20 Pages
-
Low Profile Pelvic System
16 Pages
-
Proximal Femoral (Hook) Plate
24 Pages
-
LCP
24 Pages
-
PFNA
112 Pages
-
HCS 1.5, 2.4, 3.0
36 Pages
-
LCP Wrist Fusion
32 Pages
-
LCP Compact Hand
28 Pages
-
VA-LCP Elbow
48 Pages
-
Distal Radius
44 Pages
-
Olecranon
30 Pages
-
LCP Hook Plate
28 Pages
-
DHP & Olecranon
4 Pages
-
LCP S-A
4 Pages
-
Epoca
4 Pages
-
Philos
32 Pages
-
MultiLoc
68 Pages