Website:
DKL CHAIRS GmbH
Website:
DKL CHAIRS GmbH
Catalog excerpts

EU Quality Management System Certificate (MDR) Pursuant to Regulation (EU) 2017/745 on Medical Devices, Annex IX Chapters I and III (Class IIa and Class IIb Devices) DKL CHAIRS GmbH An der Ziegelei 3 37124 Rosdorf GERMANY SRN Manufacturer - DE-MF-000005032 The Certification Body of TÜV SÜD Product Service GmbH certifies that the manufacturer has established, documented and implemented a quality management system as described in Article 10 (9) of the Regulation (EU) 2017/745 on medical devices. Details on device categories covered by the quality management system are described on the following page(s). The Report referenced below summarises the result of the assessment and includes reference to relevant CS, harmonized standards and test reports. The conformity assessment has been carried out according to Annex IX Chapter I and III of this regulation with a positive result. The quality management system assessment was accompanied by the assessment of technical documentation for devices selected on a representative basis. The certified quality management system is subject to periodical surveillance by TÜV SÜD Product Service GmbH. The surveillance assessment shall also include an assessment of the technical documentation for the device or devices concerned on the basis of further representative samples. All applicable requirements of the testing and certification regulation of TÜV SÜD Group have to be complied with. For details and certificate validity see: www.tuvsud.com/ps-cert?q=cert:G10 027377 0020 Rev. 00 Report No.: Valid from: Valid until: Issue date: Christoph Dicks Head of Certification/Notified Body TÜV SÜD Product Service GmbH is Notified Body with identification no. 0123 TÜV SÜD Product Service GmbH • Certification Body • Ridlerstraße 65 • 80339 Mu
Open the catalog to page 1
EU Quality Management System Certificate (MDR) Pursuant to Regulation (EU) 2017/745 on Medical Devices, Annex IX Chapters I and III (Class IIa and Class IIb Devices) No. G10 027377 0020 Rev. 00 Classification: Device Group: Intended Purpose: Class IIa Z12110101 - DENTAL TREATMENT UNITS - The validity of this certificate depends on conditions and/or is limited to the following: Revision History: Rev. Dated 00 2023-05-15 Description Initial issuance TÜV SÜD Product Service GmbH is Notified Body with identification no. 0123 TÜV SÜD Product Service GmbH • Certification Body • Ridlerstraße 65 •...
Open the catalog to page 2All DKL CHAIRS GmbH catalogs and technical brochures
-
Prestan Stainless Steel
6 Pages
-
Prestan Stainless Steel
16 Pages
-
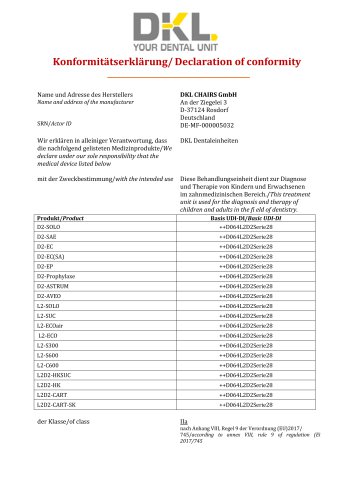
Declaration of conformity
3 Pages
-
Main Catalogue
25 Pages