
Catalog excerpts

2015 ANNUAL CLINICAL UPDATE
Open the catalog to page 1
Ovation and Ovation Prime Abdominal Stent Graft Systems 2015 Annual Clinical Update 2015 ANNUAL CLINICAL UPDATE Ovation® and Ovation Prime® Abdominal Stent Graft Systems
Open the catalog to page 2
Ovation and Ovation Prime Abdominal Stent Graft Systems 2015 Annual Clinical Update We are pleased to present the third edition of the Ovation and Ovation Prime Annual Clinical Update, which includes worldwide clinical and commercial experience. The Ovation Abdominal Stent Graft System Clinical Study was initiated in November 2009, and subjects will continue to be followed through 5 years. The Ovation device has been commercially available in Europe since CE Marking in August 2010 and in the U.S. since HDE approval in November 2011 (20 mm device) and PMA approval in October 2012 (all...
Open the catalog to page 3
Ovation and Ovation Prime Abdominal Stent Graft Systems 2015 Annual Clinical Update Device Description The Ovation and Ovation Prime Abdominal Stent Graft Systems are endovascular devices delivered via a small diameter catheter to treat abdominal aortic aneurysms (AAAs). The stent graft is a modular configuration comprised of an aortic body section, iliac limbs, and iliac extensions as required. The aortic body is comprised of a proximal stent for suprarenal fixation and a low-permeability polytetrafluoroethylene (PTFE) graft. The stent is designed with integral anchors to enable fixation...
Open the catalog to page 4
Ovation and Ovation Prime Abdominal Stent Graft Systems 2015 Annual Clinical Update Section I: Clinical Experience The data presented below include available subject information from the Ovation/Ovation Prime Abdominal Stent Graft System post-approval study cohort as of July 31, 2015. The data include Pivotal and Continued Access subjects from the Ovation clinical study and data from subjects enrolled in the PAS de novo cohort. Patient Status and Accountability Of 161 enrolled Pivotal subjects, 152 subjects were eligible for a 12-month follow-up visit, 138 were eligible for a 2 year...
Open the catalog to page 5
Data analysis sample size varies for each of the time points above and in the following tables. This variability is due to subject availability for follow-up as well as quantity and quality of images available from specific time points for evaluation. For example, the number and quality of images available for evaluation of endoleak at 6 months is different than the number and quality of images available at 12 months due to variation in the number of image exams performed, the number of images provided from the clinical site to the Core Lab, and or the number of images with acceptable...
Open the catalog to page 7
Ovation and Ovation Prime Abdominal Stent Graft Systems 2015 Annual Clinical Update Table 3. Subject and Imaging Accountability through 1-Year follow-up, de novo Data analysis sample size varies for each of the time points above and in the following tables. This variability is due to subject availability for follow-up as well as quantity and quality of images available from specific time points for evaluation. Aneurysm-related Mortality Aneurysm-related mortality (defined as death by any cause within 30 days of the index procedure) for the combined Pivotal, Continued Access, and de novo...
Open the catalog to page 8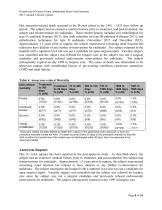
Ovation and Ovation Prime Abdominal Stent Graft Systems 2015 Annual Clinical Update One aneurysm-related death occurred in the Pivotal cohort in the 1461 - 1825 days follow up period. The subject had an extensive medical history prior to treatment, and post-treatment, the subject had reinterventions for endoleaks. These reinterventions included coil embolization for type IA endoleak (January 2011), iliac limb extension for type IB endoleak (February 2011), and embolization techniques for type II endoleaks (November 2012 and December 2012). Approximately 1.5 years prior to rupture, the...
Open the catalog to page 9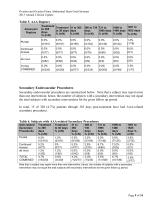
Ovation and Ovation Prime Abdominal Stent Graft Systems 2015 Annual Clinical Update Note that a subject may report more than one intervention; hence, the number of subjects with a secondary intervention may not equal the total subjects with secondary interventions for the given follow up period.
Open the catalog to page 10
Ovation and Ovation Prime Abdominal Stent Graft Systems 2015 Annual Clinical Update The following table summarizes the reasons for reintervention and the number of procedures associated with the reintervention. The type I and type III endoleaks outlined in the table were not identified by the Core Lab for several reasons, including the Core Lab categorizing the endoleak as a type II (rather than a type I) or the endoleak being identified during the reintervention and repaired (resulting in an endoleak not appearing on the follow up imaging reviewed by Core Lab). For example, in one patient,...
Open the catalog to page 11
Ovation and Ovation Prime Abdominal Stent Graft Systems 2015 Annual Clinical Update Reason for Secondary Procedure – Type IA Endoleak • Type of Secondary Procedure – Embolization 1.3% (1/77) 0.0% (0/70) 1.7% (1/60) 4.0% (1/25) Reason for Secondary Procedure – Type IB Endoleak • Type of Secondary Procedure – Stent Graft Extension 1.3% (1/77) 0.0% (0/70) 0.0% (0/60) 4.0% (1/25) • Type of Secondary Procedure – Stenting 0.0% (0/77) 0.0% (0/70) 1.7% (1/60) 0.0% (0/25) Reason for Secondary Procedure – Type II Endoleak • Type of Secondary Procedure – Embolization 3.9% (3/77) 1.4% (1/70) 3.3%...
Open the catalog to page 12
Ovation and Ovation Prime Abdominal Stent Graft Systems 2015 Annual Clinical Update The clinical trial protocol-specified CT methodology may have influenced endoleak detection rates, thereby increasing the reported rates. Slice thicknesses of 0.6 to 2.0 mm were recommended in the protocol, which were ultimately reconstructed by the imaging core laboratory to 2.0 mm for analysis. In contrast, 3-5 mm slices are commonly utilized in the follow-up of patients treated with endovascular stent grafts, which are not as effective in detecting small leaks. As the typical type II endoleak identified...
Open the catalog to page 13
Ovation and Ovation Prime Abdominal Stent Graft Systems 2015 Annual Clinical Update Data provided by site imaging. Analysis windows are: 1 month (1 to 90 days), 6 months (91 to 304 days), and 1 year (305 to 547 days). 1Denominator at each time point is the number of subjects with at least one readable scan in the time interval. 2Numerator may not equal the sum of type endoleaks if more than one type was identified in the same subject. 3Endoleaks in this section include all subjects with any type of endoleak. TriVascular conducted additional subset analyses of type II endoleaks to evaluate...
Open the catalog to page 14All Endologix catalogs and technical brochures
-
AFX® Endovascular AAA System
16 Pages
-
Ovation iX
5 Pages
-
IntuiTrak Powerlink System
4 Pages
-
AFX
6 Pages
Archived catalogs
-
IntuiTrak
4 Pages








