Website:
Eurocor
Website:
Eurocor
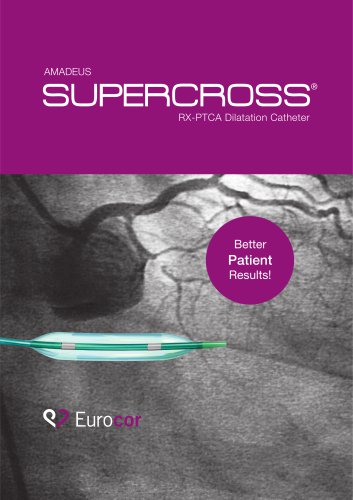
Catalog excerpts

DRUG-ELUTING PTCA BALLOON TECHNOLOGY SPECIFICALLY DESIGNED FOR CORONARY INTERVENTIONS THE COATING MAKES THE DIFFERENCE
Open the catalog to page 1
DIOR® – DRUG-ELUTING PTCA BALLOON TECHNOLOGY SPECIFICALLY DESIGNED FOR CORONARY INTERVENTIONS DIOR® – COATING CHARACTERISTICS Paclitaxel Paclitaxel is an active ingredient that inhibits the cell replication; thus blocking the microtubules decomposition during the metaphase and anaphase stages of mitosis. Shellac is a natural resin composed of shellolic and alleuritic acid. The excellent film forming properties of shellac are used to coat pharmaceutical products and in the food industry. By selectively inhibiting the proliferation of smooth muscle cells, paclitaxel does not influence...
Open the catalog to page 2
DIOR® – DRUG-ELUTING PTCA BALLOON TECHNOLOGY SPECIFICALLY DESIGNED FOR CORONARY INTERVENTIONS DIOR® – COATING CHARACTERISTICS Amorphous Coating The durable non-crystalline bioshell coating homogenously covers the balloon surface and protects the drug from mechanical abrasion and early wash off, resulting in a low paclitaxel blood plasma concentration. level of no toxicity reaction Paclitaxel blood plasma concentrations at 5 and 10 minutes after inflation (60 sec) with DIOR® DEB. After inflations of 45 sec or less no paclitaxel could be detected in the blood plasma.2 Level of toxicity for...
Open the catalog to page 3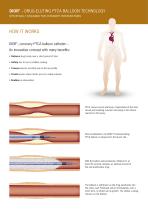
DIOR® – DRUG-ELUTING PTCA BALLOON TECHNOLOGY SPECIFICALLY DESIGNED FOR CORONARY INTERVENTIONS HOW IT WORKS DIOR®, coronary PTCA balloon catheter – An innovative concept with many benefits: • Delivers drug locally over a short period of time • Safety due to non-crystalline coating • Crosses lesions smoothly due to the low profile • Treats lesions where stents are not a viable solution • Enables re-intervention PTCA causes vessel wall injury. Hyperplasia of the inner vessel wall resulting in lumen narrowing is the natural reaction to this injury. After predilatation, the DIOR®...
Open the catalog to page 4
DIOR® – DRUG-ELUTING PTCA BALLOON TECHNOLOGY SPECIFICALLY DESIGNED FOR CORONARY INTERVENTIONS DIOR® – PRECLINICAL PROGRAM DIOR® DEB Coronary arteries of 33 domestic pig were treated with the DIOR drug eluting balloon in a time-dependent matter. Arteries were dissected and sent to a blinded laboratory for paclitaxel determination. Histomorphometry and histopathology were performed two weeks after dilatation. • No delay in endothelialization, no disadvantages in injury and inflammation score compared to standard balloon dilatation coronary arteries two weeks after dilatation. • DIOR® DEB...
Open the catalog to page 5
DIOR® – DRUG-ELUTING PTCA BALLOON TECHNOLOGY SPECIFICALLY DESIGNED FOR CORONARY INTERVENTIONS DIOR® – CLINICAL PROGRAM Evidence for the safety and efficacy of the DIOR® DEB DIOR® in in-stent restenosis (ISR) 4,5,6 Valentines Trial • International prospective multicenter registry 250 patients with coronary DES and BMS in-stent restenosis (ISR) Stella PR et al. “The Valentines Trial: results of the first one week worldwide multicentre enrolment Clinical safety and low MACE rate at follow-up in a real world ISR population. trial, evaluating the real world usage of the second generation DIOR...
Open the catalog to page 6
DIOR® - DRUG-ELUTING PTCA BALLOON TECHNOLOGY SPECIFICALLY DESIGNED FOR CORONARY INTERVENTIONS DIOR® - CLINICAL PROGRAM DIOR® in bifurcation lesions8'9 001 Trial • Prospective multicenter registry • 49 patients with coronary de novo Medina 001 bifurcation lesions • 12 months follow-up • DIOR® as a safe and technically easy option for treatment of sidebranch ostial lesions (001 bifurcations). DEBIFU Registry • Prospective multicenter registry • 100 patients with coronary bifurcation lesions 8 Vaquerizo B et al. "Second-Generation Drug-Eluting Balloon for Ostial Side Branch Lesions...
Open the catalog to page 7
DIOR® - DRUG-ELUTING PTCA BALLOON TECHNOLOGY SPECIFICALLY DESIGNED FOR CORONARY INTERVENTIONS Balloon Diameter Balloon length (mm) Eurocor Tech GmbH In den Dauen 6a, 53117 Bonn, Germany Phone: +49 (0)228/20 15 0-0 Fax: +49 (0)228/20 15 0-5 info@eurocor.de, eurocor.de Eurocor Tech GmbH is a wholly owned subsidiary of Opto Eurocor Healthcare Limited and is part of the Opto Circuits Group.
Open the catalog to page 8