Website:
Eyenuk, Inc.
Website:
Eyenuk, Inc.
Catalog excerpts

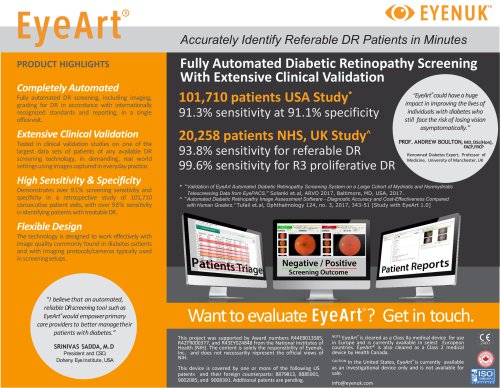
Accurately Identify Referable DR Patients in Minutes PRODUCT HIGHLIGHTS Completely Automated Fully automated DR screening, including imaging, grading for DR in accordance with internationally recognized standards and reporting, in a single officevisit. Extensive Clinical Validation Tested in clinical validation studies on one of the largest data sets of patients of any available DR screening technology, in demanding, real world settingsusing images capturedin everyday practice. High Sensitivity & Specificity Demonstrates over 91% screening sensitivity and specificity in a retrospective study of 101,710 consecutive patient visits, with over 98% sensitivity in identifying patientswith treatableDR. Fully Automated Diabetic Retinopathy Screening With Extensive Clinical Validation “EyeArt could have a huge 101,710 patients USA Study* impact in improving the lives of ® 20,258 patients NHS, UK Study^ 93.8% sensitivity for referable DR 99.6% sensitivity for R3 proliferative DR individuals with diabetes who still face the risk of losing vision asymptomatically.” PROF. ANDREW BOULTON, MD, DSc(Hon), FACP,FRCP Renowned Diabetes Expert, Professor of Medicine, University of Manchester, UK * “Validation of EyeArt Automated Diabetic Retinopathy Screening System on a Large Cohort of Mydriatic and Nonmydriatic Telescreening Data from EyePACS,” Solanki et.al, ARVO 2017, Baltimore, MD, USA, 2017. ^ “Automated Diabetic Retinopathy Image Assessment Software - Diagnostic Accuracy and Cost-Effectiveness Compared with Human Graders,” Tufail et.al, Ophthalmology 124, no. 3, 2017, 343-51 [Study with EyeArt 1.0] Flexible Design The technology is designed to work effectively with image quality commonly found in diabetes patients and with imaging protocols/cameras typically used in screeningsetups. “I believe that an automated, reliable DR screening tool such as EyeArt®would empowerprimary care providers to better managetheir patients with diabetes.” SRINIVAS SADDA, M.D President and CSO, Doheny Eye Institute, USA Want to evaluate This project was supported by Award numbers R44EB013585, R42TR000377, and R43EY024848 from the National Institutes of Health (NIH). The content is solely the responsibility of Eyenuk, Inc. and does not necessariliy represent the official views of NIH. This device is covered by one or more of the following US patents and their foreign counterparts: 8879813, 8885901, 9002085, and 9008391. Additional patents are pending. ? Get in touch. NOTE: EyeArt® is cleared as a Class IIa medical device for use in Europe and is currently available in select European countries. EyeArt® is also cleared as a Class 2 medical device by Health Canada. CAUTION: In the United States, EyeArt® is currently available as an investigational device only and is not available for sal
Open the catalog to page 1
Eyenuk Inc. headquartered in Los Angeles, California, is focused on 3/16” quickly and accurately identifying patients suffering from potentially blinding eye diseases and preserving their vision. Using computer vision and image analysis expertise, the company is developing a portfolio of products based on its proprietary retinal image analysis technology to identify and track the progression of eye diseases. COVER BACK In addition to , Eyenuk’s product portfolio includes these investigational devices: EyeMark™: Accurately track retinopathy progression with image-based biomarkers. 3/16”...
Open the catalog to page 2All Eyenuk catalogs and technical brochures
-
EyeArt® Presentation
17 Pages