Website:
Eyenuk, Inc.
Website:
Eyenuk, Inc.
Catalog excerpts

Cloud-based Retinal Image Analysis Software kS>6P»to, !4 6 RW.oipi-q 1APJ kjG$ftdl ffeQficvi.qiw^ ? j/jvoa «rio fiMrfn o Uq I q i L Fully Automated, Point of Care, i i.u.i j ,;i OO "1 p^ni IO 11 1 Diabetic Retinopathy Screening i 'Ctvc’raysi: ipnotOTitoa| >1 imjnrfe.cnj jmrtl 1 aojcsoti oscoiaeotn Hrn:i aarin v in a t so ■trvs o to to 5®r tro i nnm v ot it a tj^^ltl-QiliQiLScftqbaotiDrol Q1- 1Is: 'I liQV .ao-ilMifii/fcsWDna1*r-o <r o 1* a o i:tpr- i ott10 i^ 1:mi< BRSbtfl’It'-'tiQaiDOaOoiaO'lkOcjOrInrtJnliiO'.l CM-. In • O : omo 11 i 10101 1 0101 1 10
Open the catalog to page 1
MISSION Accelerating world’s transition to predictive and preventative healthcare with innovative AI-driven diagnostic solutions. VISION Develop robust products with most advanced healthcare technology and make them accessible to everyone. “Screen every eye in the world” Sept 2018
Open the catalog to page 2
Hypertension / Stroke Hypertension / Stroke Cardiovascular Diseases Powerful biomarkers for target diseases through retinal analysis ^5 ^*ony%rganlwil aitoWs^oniinViiiiiiViiWing^and^imaginpSimicrosViicylature 7^^
Open the catalog to page 3
Where technology meets real needs Approved in Europe and Canada. Anticipated FDA in early 2019. Prospective clinical trials enrollment complete. has been cleared for sales as a Class IIa medical device by EU and as a Class 2 medical device by Health Canada. In the United States, is limited by federal law to investigational use only and is not available for sale. Automated detection and grading of diabetic retinopathy (DR)
Open the catalog to page 4
Easy to understand screening results. PDF diagnostic report generated. Triage at the point of care. Patients who screen positive immediately referred to eye specialists. Retinal images taken using a fundus camera. Non invasive, and no dilation required. Images are uploaded to EyeArt Cloud. Secure, encrypted, HIPAA-compliant transfer. Automated analysis and secure reporting in 60 seconds. No human grading required. is fast and easy to use, and provides clear actionable output.
Open the catalog to page 5
Summary No signs of rDED (moderate NPDR, severe NPDR, PDR, or surrogate markers for CSME) are likely present in the patient's fundus photographs input to Negative for rDED Click to export results -RIGHT EYE [OD]- Referable DED Vision Threatening DED rDED = Referable Diabetic Eye DiseaseSept 2018
Open the catalog to page 6
Increased Efficiency Current retinal screening Eyenuk solution Requires specialist to read images or perform direct ophthalmoscopy Any PCP/GP or technician can perform 2-6 months typical wait to see ophthalmologist Patient screened during annual physical/check-up Hard to scale due to lack of specialists qualified to read images Cloud-based software “reads” images in 60 seconds or less Dilation is necessary (in case of direct ophthalmoscopy) Dilation most likely not needed.
Open the catalog to page 7
Elevated clinical quality Improved screening accuracy Current practice Sensitivity of direct ophthalmoscopy in screening can be quite low (~ 65%) With EyeArt EyeArt shown to have 91% sensitivity/ 91% specificity in over 100K patient visits. Harding et al., “Sensitivity and Specificity of Photography and Direct Ophthalmoscopy in Screening for Sight Threatening Eye Disease: The Liverpool Diabetic Eye Study.” 1995, BMJ 311 (7013): 1131–35. h Lin et al. “The Sensitivity and Specificity of Single-Field Nonmydriatic Monochromatic Digital Fundus Photography with Remote Image Interpretation for...
Open the catalog to page 8
Elevated clinical quality Imaging, Grading for DR, and Reporting in a Single Office Visit. Current practice Image read can take a day or up to a week in most telemedicine programs. With EyeArt Designed to analyze all patient images under 60s. Increased patient compliance expected with immediate reporting. Daskivich et al. “Implementation and Evaluation of a Large-Scale Teleretinal Diabetic Retinopathy Screening Program in the Los Angeles County Department of Health Services.” 2017, JAMA Internal Medicine, March.
Open the catalog to page 9
Elevated clinical quality Consistently accurate Current practice With EyeArt Human grading can vary based EyeArt is consistent by design on expertise, training, and is highly repeatable. fatigue, and other factors. Ruamviboonsuk et al. “Interobserver Agreement in the Interpretation of Single-Field Digital Fundus Images for Diabetic Retinopathy Screening.” 2006, Ophthalmology 113 (5): 826–32. Patra et al. “Interobserver Agreement between Primary Graders and an Expert Grader in the Bristol and Weston Diabetic Retinopathy Screening Programme: A Quality Assurance Audit.” 2009 Diabetic Medicine...
Open the catalog to page 10
Challenges faced Patient How to improve patient triage and provide access to quality eye care with limited specialist resources? How do I keep my eyesight intact and know when treatment is needed? How to reduce cost of eyecare related to diabetes (and other conditions) and reduce incidence of expensive eye complications? 11
Open the catalog to page 11
How EyeArt can help Patient • Preventing vision loss • Improved patient experience and satisfaction • Improved screening capacity and patient triage • Better utilization of specialist resources • Reduced per-patient operating costs • Early diagnosis for prevention of high cost complications • No specialist costs for patients with Negative
Open the catalog to page 12
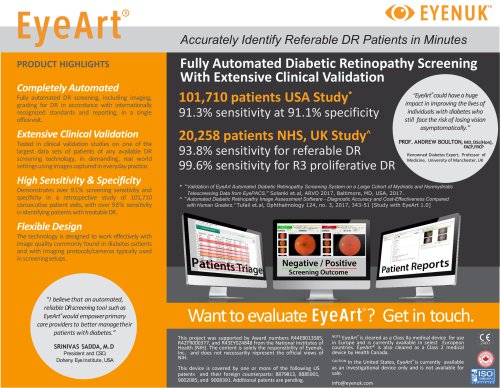
Moorfields Eye Hospital NHS Foundation Trust EyeArt clinical validation studies NHS Moorfields Study (Tufail et al. Ophthalmology 2017) 20,258 patients, consecutive, independent, retrospective 93.8% sensitivity for referable DR; 99.6% sensitivity for proliferative DR Sensitivity, false positive rates not affected by ethnicity, gender, or camera type 100K Patient Study (Solanki et al. ARVO 2017) Retrospective study of 107,001 consecutive patient visits from 404 clinics Multiple cameras/large image quality variation 91% sensitivity and 91% specificity for referable DR screening Study with...
Open the catalog to page 13
Independent, observational study* of 20,258 consecutive patients comparing three automated DR screening systems: EyeArt, Retmarker, iGrading Conclusions: Compared to quality assured manual grading, EyeArt v1.0 achieves • Acceptable sensitivity for referable DR • Sufficient specificity to make it a cost effective alternative Sensitivity and false positive rates for EyeArt were not affected by ethnicity, gender, or camera type Detection criteria HEALTH TECHNOLOGY ASSESSMENT VOLUME 20 ISSUE 92 DECEMBER 2016 An observational study to assess if automated diabetic retinopathy image assessment...
Open the catalog to page 14All Eyenuk catalogs and technical brochures
-
EyeArt® Brochure
2 Pages
-
EyeArt® Presentation
17 Pages