Website:
Eyenuk, Inc.
Website:
Eyenuk, Inc.
Catalog excerpts

Cloud-based Retinal Image Analysis Software kS>6P»to, !4 6 RW.oipi-q 1APJ kjG$ftdl ffeQficvi.qiw^ ? j/jvoa «rio fiMrfn o Uq I q i L Fully Automated, Point of Care, i i.u.i j ,;i OO "1 p^ni IO 11 1 Diabetic Retinopathy Screening i 'Ctvc’raysi: ipnotOTitoa| >1 imjnrfe.cnj jmrtl 1 aojcsoti oscoiaeotn Hrn:i aarin v in a t so ■trvs o to to 5®r tro i nnm v ot it a tj^^ltl-QiliQiLScftqbaotiDrol Q1- 1Is: 'I liQV .ao-ilMifii/fcsWDna1*r-o <r o 1* a o i:tpr- i ott10 i^ 1:mi< BRSbtfl’It'-'tiQaiDOaOoiaO'lkOcjOrInrtJnliiO'.l CM-. In • O : omo 11 i 10101 1 0101 1 10
Open the catalog to page 1
United States Fundus image Screening with Severity Grades Automated Diabetic Retinopathy Screening Software Cutting-edge Image Analysis and Artificial Intelligence (AI) packaged into a Cloud-based SaaS (Software as a Service).
Open the catalog to page 2
Where technology meets real needs Approved in Europe and Canada. Anticipated FDA in early 2019. Prospective clinical trials enrollment complete. has been cleared for sales as a Class IIa medical device by EU and as a Class 2 medical device by Health Canada. In the United States, is limited by federal law to investigational use only and is not available for sale. Automated detection and grading of diabetic retinopathy (DR)
Open the catalog to page 3
Easy to understand screening results. PDF diagnostic report generated. Triage at the point of care. Patients who screen positive immediately referred to eye care specialists. Retinal images taken using a fundus camera. Noninvasive and no dilation required. Images are uploaded to EyeArt Cloud. Secure, encrypted, HIPAA-compliant transfer. Automated analysis and secure reporting in 60 seconds. No human grading required. is fast and easy to use, and provides clear actionable output.
Open the catalog to page 4
Negative for rDED Click to export results Summary No signs of rDED (moderate NPDR, severe NPDR, PDR, or surrogate markers for CSME) are likely present in the patient's fundus photographs input to Positive for rDED Click to export results Signs of proliferative DR and signs of surrogate markers for CSME are likely present in the patients fundus photographs input to EyeArt. CSME Surrogate Markers CSME Surrogate Markers Possibly Absent Possibly Absent LEFT EYE [OS] Possibly no DR i_J_f_ CSME Surrogate Markers CSME Surrogate Markers Possibly Severe NPDR Possibly Present Possibly Present *rDED =...
Open the catalog to page 5
Rapid and reliable output (report) Retinal images of both eyes, which have been captured by compatible fundus cameras, are uploaded to the secure cloud-based platform for processing. automatically analyzes the images and returns the result in less than 60 sec. report contains positive/negative DR screening outcome, clear indication of image quality, DR severity level, and retinal images.
Open the catalog to page 6
AUTOMATED DR SCREENING USING AI *0622670' Negative for rDED Positive for rDED IPatteat 1852 2fol I 852 2ft»l_36 I Unspecified Summary Signs of rDED (moderate NPDR, severe NPOR, PDR, or surrogate markers for Negative / PositiveScreening Outcome Patient Report* <•> EYENUK
Open the catalog to page 7
provides for simple, per-use, model allowing friction-less adoption. The technology enables scalable, highly accurate, and cost effective mass screening with fewer human resources. makes setting-up of DR screening programs easy and cost effective.
Open the catalog to page 8
PATIENTS AFFECTED BY DIABETES WORLDWIDE A Community that needs a response Patients affected worldwide Annual US healthcare costs due to DR Costly manual procedures
Open the catalog to page 9
SCREEN EVERY EYE IN THE WORLD O u r Vi si on Develop robust products with the most advanced healthcare technology and make them accessible to everyone.
Open the catalog to page 10
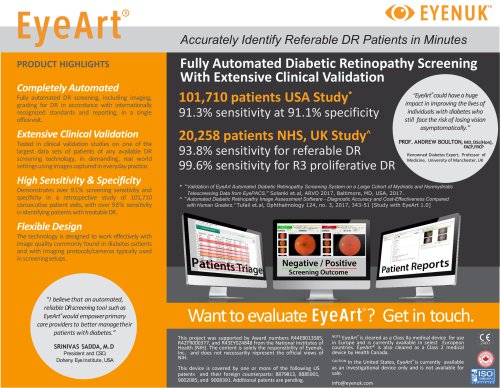
Unparalleled clinical evidence Technology proven in very large studies PATIENT VISITS PATIENT VISITS SENSITIVITY AND USA STUDY NHS UK STUDY 100K+ PATIENTS • 91% sensitivity and 91% specificity in over 100K patient visits • Results comparable to human graders in a fraction of the time and cost • Compliant with internationally recognized clinical standards • Available 24/7: can process exams in large batches
Open the catalog to page 11
Moorfields Eye Hospital NHS Foundation Trust EyeArt clinical validation studies NHS Moorfields Study (Tufail et al. Ophthalmology 2017) 20,258 patients, consecutive, independent, retrospective 93.8% sensitivity for referable DR; 99.6% sensitivity for proliferative DR Sensitivity, false positive rates not affected by ethnicity, gender, or camera type 100K Patient Study (Solanki et al. ARVO 2017) Retrospective study of 107,001 consecutive patient visits from 404 clinics Multiple cameras with large image quality variation 91% sensitivity and 91% specificity for referable DR screening Study...
Open the catalog to page 12
WE ARE ON THE RIGHT PATH Global presence
Open the catalog to page 13
...EyeArt achieved acceptable sensitivity for referable retinopathy and false positive rates (compared to human graders as reference standard).” '] : * Li i “.An important milestone towards getting automated screening for diabetic retinopathy.” “.We have examined your results and found a good agreement with ^ — _ __ “.Importantly, the (EyeArt) automated system did not miss ANY of the referable retinopathies in 10,000 f - ‘• • -.3 • . ’ • *; . ’v . consecutive screens.” OPHTHALMOLOGISTS OPTOMETRISTS DIABETOLOGIST
Open the catalog to page 15
CONTACT US HARNESSING DEEP LEARNING TO PREVENT BLINDNESS START SCREENING CONTACT US TEST OUR SOFTWARE EYENUK.COM info@eyenuk.com AMERICAS □ EYENUK, INC. 5850 CANOGA AVE, STE 250 LOS ANGELES, CA 91367, USA PHONE : + ( 1 ) 818 835 3585 □ EYENUK EUROPE LTD WEST WING 5 , SUITE 21 THE QUADRANGLE. CREWE HALL, WESTON ROAD. CREWE, CHESHIRE. CW1 6UZ. UK PHONE : + ( 44) ( 0) 7 47 08 6 8 967 EyeArt™ has been cleared for sales as a Class I la medical device by EU and as a Class 2 medical device by Health Canada. In the United States, EyeArt* is limited by federal law to investigational use only and...
Open the catalog to page 17All Eyenuk catalogs and technical brochures
-
EyeArt® Brochure
2 Pages