Catalog excerpts

Application Case Study → Laser Marking Dental Products Technologically advanced, small in size, greatly effective Direct marking is required for reusable, reprocessed dental devices (such as instruments)*; however, many manufacturers of singleuse or general-use dental products – ranging from implants to complete practice equipment – highly value the many production, product and treatment related benefits as well as the competitive advantages permanent laser marking of their products provides. Products and Applications Dental products are marked for various reasons with various content. The UDI labelling regulation* applies to multi-use instruments that have to be reprocessed (e.g. autoclave) before each use. These include endodontic and parodontic instruments, various curettes and sinus lift instruments, implant drills, osteotomes and condensers. Instruments like these also have to be marked with permanent CE signs or – as in the case of drills – with depth gauges. Direct marking does not apply to sterile packed dental implants and screws. However, for many reasons, it makes good sense to apply a direct laser mark on such dental products, and many manufacturers already do so: Brand logos, trademark signs and the CE sign ensure protection against counterfeiting; laser marked depth gauges provide treatment safety; and reliably machine-readable 2D codes guarantee full traceability throughout the entire product lifecycle. Challenges Besides the various regulations – such as the UDI directive – hospital logistics related requirements are increasingly gaining importance. Clinics benefit from standardized, consistently marked and reliably traceable dental products. Manufacturers providing products in accordance with these demands enjoy distinct competitive advantages. Additionally, also functional markings such as depth gauges or optical markings for the purpose of brand and counterfeit protection are required. High quality standards, patient safety and traceability have to be ensured as well as an efficient and almost error-free production with as little scrap as possible. Furthermore, next to the materials – ranging from Titanium nitride through stainless to Titan-oxidized surfaces as well as some plastic compounds –, it's the direct marking of the * In 2018, reprocessed Class II devices that are distributed in the US have to directly carry a permanent UDI code. For multi-use Class I products, a directly marked UDI code is compulsory as of 2020. In Europe, multi-use Class IIa/IIb products have to carry the UDI code as of 2025 and reusable Class I products as of 2027 according to the new MDR (Medical Device Regulation). Why it's beneficial to laser mark dental products → regulatory labelling requirements (e.g. UDI) → functional requirements (e.g. depth gauges) → quality assurance → patient safety → brand/forgery protection → trac
Open the catalog to page 1
Application Case Study: Laser Marking of Dental Products tiny devices that challenges the industry. The smallest markings have to be applied in minimal spaces with the highest resolution and contrast. All marks must be biocompatible and reliably readable. In the case of reusable reprocessed instruments, all marks have to survive approximately ten to twenty autoclave cycles. The Solution Cost-effective dental device marking thanks to integrated vision FOBA's laser marking solutions are ideally suited for the reliable, permanent and economic identification of dental devices. Vision systems...
Open the catalog to page 2All FOBA Laser Marking + Engraving catalogs and technical brochures
-
Software and Vision overview
7 Pages
-
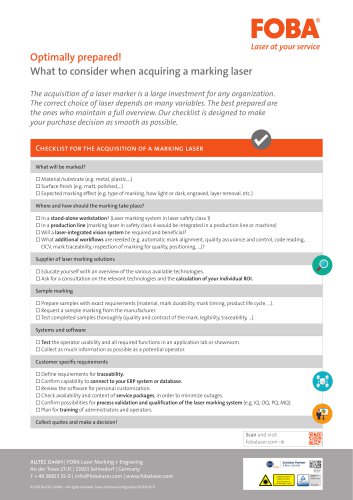
Laser purchase infographic
1 Pages
-
Service Packs Overview
4 Pages
-
Product brochure
4 Pages