Catalog excerpts

Medical Industry White Paper Unique Device Identification (UDI) Requirements, deadlines, secure labeling according to FDA and MDR The implementation of the UDI Directive is mandated for medical device manufacturers. This has been in effect since 2014 for companies selling their products on the American market, and, as of 2020, also in the EU and other countries. Medical products must be clearly identified to ensure reliable traceability and patient safety. In addition to the marking of packaging and labels, this also applies to the direct marking of high risk devices such as implants and instruments. Manufacturers must maintain deadlines and need to implement marking systems that are able to mark in accordance with UDI requirements and medical standards. In order to fulfill the demanding labeling, serialization and marking requirements, appropriate technologies must allow for the application of high resolution codes with long-term durability, even in the smallest of spaces, while additionally providing a verification process to read back code contents and quality. Laser marked UDI codes
Open the catalog to page 1
The UDI program: effective since 2014 in the USA Consistent labeling of medical devices for an increase in patient safety and transparency in the supply chain In 2013, the FDA (Food and Drug Administration) released the UDI Directive for the uniform labeling of medical devices with the marking specification in its "final rule". Standardized labeling is meant to enable the complete identification and tracking of medical devices throughout the entire product life cycle. In 2017, the European Union and other countries closed the gap and implemented an equivalent, the Medical Device Regulation...
Open the catalog to page 2
UDI in the European Union – Medical Device Regulation (MDR) "System of a unique product number" as of 2020 In the EU, the UDI directive has been introduced as a "Unique Device Identification"* system within the framework of the new European Medical Device Regulation (Medical Device Regulation - MDR) in 2017. After a transition period of three to five years, the MDR will become effective in stages for all risk classes as of May 26, 2020.* * Regulation 2017-745 on medical devices of 5 April 2017 (versions in English and other languages) → https://eur-lex.europa.eu/eli/reg/2017/745/oj The UDI...
Open the catalog to page 3
UDI codes on packaging or as direct marking: Risk classes determine compliance dates Where and when a UDI code has to be applied to a product depends on its nature (risk class) and intended use. The UDI system differentiates between the marking of the packaging or labels and direct marking on the product. According to the FDA, the last deadline for the marking of packaging and labels had been reached in September 2018. UDI direct part marking for all risk classes in the USA will be due at latest by September 2022. According to the MDR, direct marking will not be mandatory immediately on the...
Open the catalog to page 4
bandages stethoscopes surgical scissors dental floss mechanical wheelchairs Infusion pumps surgical sutures bone screws syringes condoms powered wheelchairs heart valves knee prosthesis pacemakers automated external defibrillators low risk, general controls No directly marked UDI is required for sterile packaged implants that are unpacked directly at the implant site. A directly marked UDI code is only required if the corresponding products are processed (for example, cleaned, sterilized or passivated) before their use. Instruments that are repeatedly used and reprocessed must - depending...
Open the catalog to page 5
FDA and MDR compliance dates All deadlines at a glanceThe UDI system is phased in stages based on product classification risk level, starting with the products with the highest risk class. Find below the labeling deadlines for products manufactured in or imported into the United States.Deadline UDI Labeling obligations apply to ... September 24, 2014 Source: Compliance Dates for UDI requirements (FDA) http://www.fda.qov/MedicalDevices/DeviceRequlationandGuidance/ UniqueDeviceldentification/CompliancedatesforUDIRequirements/default.htm List of medical devices that FDA classifies as...
Open the catalog to page 6
There is a transition period of up to four years after the European MDR enters into force, i. e. until May 25, 2024 the hitherto MDD (Medical Device Directory) is still valid. Find hereafter the UDI marking compliance dates for products that are manufactured in or exported to the EU (according to art. 123 para. 3 f-h MDR): See the Official Journal of the European Union online: "REGULATION (EU) 2017/745 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 5 April 2017 on medical devices, amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing...
Open the catalog to page 7
Reliable and unique: Code format and structure of a UDI code Clear regulations apply for UDI codes, since only a uniform code format and reliably readable marks and labels ensure reliable traceability. According to 21 CFR 81.40, medical devices have to carry a UDI that is represented in two forms: → Easily readable plain-text (HRI/Human Readable Interpretation), alpha-numeric characters → AIDC technology (Automatic Identification and Data Capture), which means a machine readable code (bar code/2D code). In case the UDI code is applied as a permanent direct mark, it must be in either or...
Open the catalog to page 8
Code format: UDI = DI + PI The Unique Device Identifier consists of two parts, the Device Identifier (DI) and the Production Identifier (PI). An exception are Class I medical devices, for which the labeling with the Production Identifier is not required. The Device Identifier (DI) is the mandatory, fixed portion of a UDI that identifies the labeler and the device (specific version or model). The DI contains the following static information: Identifies the manufacturer/labeler Specific version or model of device (also reference code) This information serves as the key to obtain device...
Open the catalog to page 9
Coding system and issuing agency: International formatting standards for UDI codes UDI coding for medical products does not implement a new code format, but uses existing standards. Machine readable linear barcodes are in use as well as DataMatrix Codes, both following international standards and enabling product identification throughout the entire product life cycle (see MDR, Art. 27, para. 2). Both, the Health Industry Barcode (HIBC) and the GS1 code are equally suitable for the UDI compliant marking of medical products and devices. With the ISBT 128, the ICCBBA offers a special system...
Open the catalog to page 10All FOBA Laser Marking + Engraving catalogs and technical brochures
-
Software and Vision overview
7 Pages
-
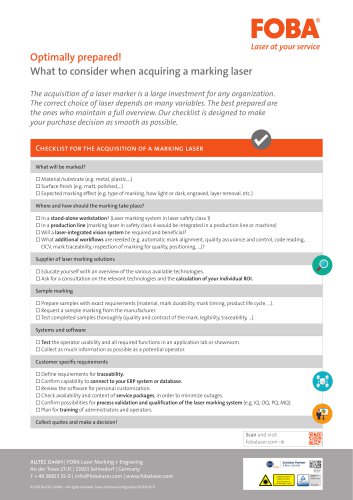
Laser purchase infographic
1 Pages
-
Service Packs Overview
4 Pages
-
Product brochure
4 Pages