 Website:
GenSci Group
Website:
GenSci Group
Catalog excerpts

GeneScience Pharmaceutical Co., Ltd. All right reserved©2024 GeneScience
Open the catalog to page 1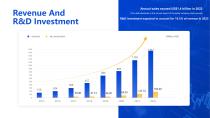
Revenue And R&D Investment REVENUE Annual sales exceed US$1.4 billion in 2022 (The data disclosed in the annual report of the listed company shall prevail) R&D investment expected to account for 16.5% of revenue in 2023 Expected to account for 16.5% of revenue in 2023 Million USD
Open the catalog to page 2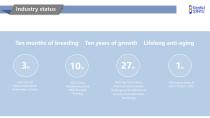
Industry status 2022 Top 20 Biopharmaceutical Enterprises in China 2023 China Biopharmaceutical R&D Strength Ranking Lifelong anti-aging 2022 Top 100 Chinese Pharmaceutical Industry Rankings by the Ministry of Industry and Information Technology 76% market share of GH in China in 2023
Open the catalog to page 3
Injection pen product capability With a number of independent intellectual property rights, With the domestic exclusive growth hormone electronic injection pen, easy to operate, high precision, can effectively reduce pain. thousands of injection pen patents, no infringement risk. high patent freedom, and the formation of patent barriers. stimulating hormone and other drugs, FTO analysis of tens of ✓ Core members from Novo Nordisk, Sanofi, YPS and other Kinsey growth hormone accounted for 76% of the pediatric market, and injection pens were sold for nearly ten years. With more than 10 years...
Open the catalog to page 4
✓ production capacity • Established a 2500 square meter fully ✓ Standard regulations • ISO 11608 Needle based injection systems automated intelligent manufacturing factory for medical use series standards YY/T 1768 Series of Requirements and Test Methods Systems • ✓ Quality Control&Quality System • The qualified rate of products is • 100% The qualified rate of primary quality is more than 98% for Pen, Jet, and Related Injectors Intended for use with Drugs and Biological Products • YBB Pen Syringe Series Standard • Shanghai "Intelligent Factory Demonstration" Project ISO9001、ISO13485、...
Open the catalog to page 5
Pen length is 15% shorter than YPS, lighter in weight, and more environmentally friendly No“chlorine”spring, not easy to break, not rusty During injection, the screw moves straight and is not easily deformed Innovative residual dose protection function, more reliable structure Dose adjustment sound prompt and clear indication Appearance customization Applicable to drugs with a filling volume of 0.2-1.0mL Equipped with automatic needle insertion (including automatic unlocking function) Needle penetration depth of 5-8mm Featuring a medication viewing window Sound and visual prompts for...
Open the catalog to page 6
Pipeline of Disposable/Reusable Injection pen Model Cartridge Type Needle Type Dose division Adjustable dosage (dual-direction regulation) Remaining dose projection Dose setting✔ Dose reversal✔ Dose delivery✔ Audio feedback External dimensions
Open the catalog to page 7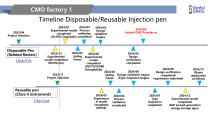
Timeline Disposable/Reusable Injection pen 2024/01 2024/01 Experimental model Process validation completed (DLP30 Liraglutide)completed Disposable Pen (Related Review) 2023/12 Experimental model completed (DLP60 pen) Reusable pen (Class II instrument) 2024/02 Design validation begins 2024/01 2024/02 styling Experimental freeze model completed (DLP75/DLP80 Semaglutide) 2024/05 Design verification completed 2024/04 2024/04 styling Design validation begins /Type inspection begins freeze 2024/03 Experiment al model completed (RLP60) 2024/04 Process validation completed 2024/08 Design...
Open the catalog to page 8
Production capacity 4000 square meters of industrial plan With automated assembly lines, continuous and stable production is possible Advantages The unique manufacturer in China that can produce cases of high-dose and low-dose mechanical pens Maximum injection dose: 800 μ L Quality system The production process is fully managed in accordance with Standard regulations ISO 11608 Needle based injection systems for medical use series standards YY/T 1768 Series of Requirements and Test Methods for Medical Needle Injection Systems FDA-2009-D-0179 Technical Considerations for Pen, Jet, and Related...
Open the catalog to page 9
Competitive Advantage with foreign competitors Development efficiency Development expenses Project Changes Quick response Patent risk Quality awareness Manual/automatic line Manual/automatic line Manual/automatic line Manual/automatic line Manual/automatic line More than 3 months Supply efficiency More than 3 months More than 3 months More than 3 months
Open the catalog to page 11
Competitive Advantage with domestic competitors Development efficiency Development expenses 800 thousand-1 million 800 thousand-1 million Project Changes Compatible response Compatible response Compatible response Compatible response Quick response Patent risk Quality awareness Manual/automatic line Supply efficiency
Open the catalog to page 12All GenSci Group catalogs and technical brochures
-
GenSci auto-injector
2 Pages
-
GenSci introduction
26 Pages



