 Website:
Gore
Website:
Gore
Catalog excerpts

Greater options PERFORMANCE through innovation The Continuing Evolution of a Revolutionary Device
Open the catalog to page 1
GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface introduced in US Original GORE® HEMOBAHN® Endoprosthesis introduced in Europe 2007 2009 Laser technology enables the new contoured edge at proximal end 5 – 8 mm devices decreased in profile by one French size 9 – 13 mm devices introduced with 0.035˝ guidewire compatibility 2011 GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface 5 – 8 mm devices decreased in profile by one French size 2003 Original device introduced in US as GORE® VIABAHN® Endoprosthesis TIP to HUB deployment introduced on 6 – 8 mm devices 6 – 8 mm...
Open the catalog to page 2
The Endoluminal Bypass ePTFE Lining Provides barrier to in-stent restenosis Nitinol Stent Unique stent-graft design is conformable and durable in the dynamic SFA CARMEDA® BioActive Surface (CBAS® Surface) Intended to provide thromboresistant surface Contoured Proximal Edge May improve flow dynamics as blood enters endoprosthesis Streamlined Deployment for All Configurations: 5 – 13 mm diameters • Radial endoprosthesis expansion
Open the catalog to page 3
CARMEDA® BioActive Surface (CBAS® Surface) • Intended to provide a thromboresistant surface Sustained bioactivity* Proprietary end-point covalent bonding GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface Control Endoprosthesis The bioactive luminal surface of a 5 mm diameter GORE® VIABAHN® Endoprosthesis with Heparin Bioactive Surface appears free of thrombus after two hours in an in vitro blood loop model. The non-bioactive luminal surface of a control endoprosthesis (5 mm diameter) appears covered with thrombus after 90 minutes in the same blood loop model (data on file). Mean...
Open the catalog to page 4
Proprietary End-Point Covalent Bonding Only the end of the heparin molecule is bonded to the endoprosthesis surface • The heparin bioactive sites remain free to interact with the blood • Heparin molecules are bonded to the endoprosthesis surface • Bioactive site of the heparin molecule binds to antithrombin (AT) • Antithrombin binds to thrombin (T) – a neutral AT-T complex is formed AT-T • Thrombin loses its ability to catalyze the conversion of fibrinogen to fibrin • Neutral AT-T complex detaches from the heparin molecule AT-T • Heparin bioactive site becomes available to again bind...
Open the catalog to page 5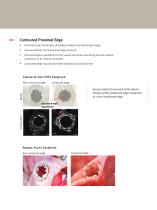
Contoured Proximal Edge • Precision laser trimming technology enables manufacturing change Excess material at the proximal edge removed Improves device apposition to the vessel wall when oversizing prevents device expansion to its nominal diameter Contoured edge may improve flow dynamics at proximal end Canine In Vivo IVUS Examples Non-contoured edge Contoured edge Excess material removed at the device margin of the contoured edge compared to a non-contoured edge improved wall apposition Animal Acute Examples Non-contoured edge Contoured edge
Open the catalog to page 6
W. L. Gore & Associates, Inc. Flagstaff, AZ 86004 +65.67332882 (Asia Pacific) 00800.6334.4673 (Europe) 800.437.8181 (United States) 928.779.2771 (United States) INTENDED USE / INDICATIONS The GORE® VIABAHN® Endoprosthesis is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in superficial femoral artery lesions with reference vessel diameters ranging from 4.0 – 7.5 mm. The GORE® VIABAHN® Endoprosthesis is indicated for improving blood flow in patients with symptomatic peripheral arterial disease in iliac artery lesions with reference vessel...
Open the catalog to page 8All Gore catalogs and technical brochures
-
Decades of data
2 Pages
-
CONFIGURATIONS
2 Pages
-
Care and Cannulation
9 Pages
-
Introducing
4 Pages
-
ADULT IC CARDIOFORM BROCHURE
8 Pages
-
Brochure Product
4 Pages
-
Pediatric Patient Booklet
36 Pages
-
GORE TIPS Needle
4 Pages
-
GORE® VIABIL®
4 Pages
-
Endovascular
28 Pages
-
GORE-TEX®
4 Pages
-
GORE® DUALMESH® PLUS
6 Pages
-
GORE® SYNECOR Biomaterial
1 Pages
-
GORE® SYNECOR
8 Pages
-
GORE-TEX® Suture
6 Pages
-
Cardiothoracic Portfolio
8 Pages
-
Hemodynamic Flow Brochure
4 Pages
-
Product Brochure
4 Pages
-
GORE 3D Imaging System
8 Pages
-
GORE® VIABAHN®
24 Pages
-
GORE® SEAMGUARD®
5 Pages
































