
Catalog excerpts

im[ swiss dental implants Component list
Open the catalog to page 1
The Swiss company IML SA Swiss Dental Implants was founded in 2009 by the synergy of professionals of the dental implantology field, creating a close-knit team with twentyyear experience in the design and production of implant systems with the highest quality standards. The IML team continuously strives to find effective solutions for new implantology needs, ones that meet the expectations of the most demanding professionals. Main aim: to offer oral implantology that is Simple, Safe and Stable through time. These “3Ss” summarise the guidelines the Company has established for its own...
Open the catalog to page 2
M e n, m a te r i a l s a nd m a c h i n es Only the best raw materials, the most advanced technology, and the best professionals. These secrets of IML guarantee excellent products, free from manufacturing defects. • Skilled operators able to develop a man-machine relationship able to optimise the features of their tools to achieve maximum performances • Top quality titanium for medical use. grade 4 for implants and grade 5 for prosthetic parts. IML titanium is exclusively imported from the United States, is guaranteed free from manufacturing defects and radioactivity • Mechanical...
Open the catalog to page 3
POWE R a nd POWE R O M I M P LA NT SYSTE MS The perfect solution for: • any bone density • post extraction • system with abutment tilt lower than 30° • electric-welded implantology with immediate loading Abutment resistant to transverse loads Hexagonal head: for connection with the implant driver Controlled finish surface roughness and polished: best aesthetic result for crown transparency Monoblock Double alternate spur and square spiral SL surface treatment Root-shaped body Self-tapping apex and rounded tip Retention channels: perfect crown anchorage IMPLANT Ø3.4, Ø4, Ø5, Ø6 Pre-defined...
Open the catalog to page 4
The biomechanical principles of the Universe system have been applied to the POWER and POWER OM implants in order to offer the implant surgeon a greater chance of solving implant-prosthetic problems by using a monophase technique. The POWER and POWER OM implants are made of top quality grade 4 titanium for medical use exclusively imported from the United States and guaranteed free of manufacturing defects. • Root-shaped body: fast, safe and minimally invasive insertion • Self-tapping screw tip • Double alternate spur and square spiral: bio-functional load distribution • SL surface...
Open the catalog to page 5
I n t ra os se o us p o r t i o n The tapered morphology of Power and its innovative twin-spiral thread, which is also present in the apical part, ensure rapid, safe, and minimally invasive surgical insertion. The double alternate spur and square spiral generates a perfect balance between intrusive, compressive, and diverging forces capable of providing the bone with exceptional growth stimuli. The immediate result of this geometric combination is high primary stability even in situations with altered bone which creates optimum conditions for an intimate contact with the bone, an advantage...
Open the catalog to page 6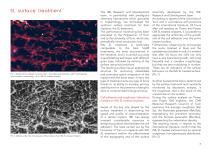
Pic 1. Details of the cellular microstructure - branched and dendritic, with long filopodia and complex morphology - of an IML SL treated implant. The IML Research and Development team, in partnership with prestigious chemistry laboratories which specialise in implantology, has formulated the optimum surface treatment for their implants: the SL treatment. The performance monitoring has been entrusted to the Polytechnic of Turin and to the University of Turin, which also periodically check production lots. IML SL treatment is technically comparable to the best SLA® treatments, the most...
Open the catalog to page 7
Even the decontamination process used for IML implants was developed in collaboration with the Research and Development team of our prestigious Italian universities partners. This is a two-stage process, the second stage being composed of passing the implants through a plasma reactor. The "PLASMA REACTOR" project aimed to build a machine with suitable characteristics for treating dental implants and to define the optimal operating procedure and was conducted in close co-operation with the Department of Applied Science and Technology of the Polytechnic University of Turin and the Department...
Open the catalog to page 8
S u rg i ca l K i t The Power surgical box is made entirely of plastic materials suitable for steam sterilisation. The instrument positions are clearly labelled in order to facilitate identification during the surgical operation. ripiano sottostante The silicon supports secure the instruments firmly during transportation and sterilisation. Drill stops are supplied in two separate kits based on the drill diameter. Both drill stops and drills are immediately identifiable thanks to the color code: the drill stops are anodized, while on the drills there is a colored o-ring. In particular: RED...
Open the catalog to page 10
Cylindrical pilot drill Drill extension Red drill stops kit for drills 02-2.7-3.1-3.6-4 Green drill stop for drills 04.4-5.3 Cylindrical drill
Open the catalog to page 11
Implant driver for motor Fixed ratchet Multitool implant driver Multitool manual driver Dynamometric ratchet
Open the catalog to page 12
Precision drill Precision drill The Power surgical protocol was developed to provide surgeons with indications on how to choose the most suitable instruments for implant site preparation, depending on the type of bone. However, it is the duty of the surgeon to apply the most appropriate surgical protocol on the basis of his/her experience and following a thorough assessment of the clinical situation of the individual patient. For the preparation of the implant site, IML has developed cylindrical drills with a tapered tip and depth marks in accordance with the length of the implant; they can...
Open the catalog to page 17
Pa c ka g i n g IML packaging process is performed in compliance with the standards set by the MDR 2017/745 Directive, which guarantee the sterilisation shelf-life. All the IML implants are sterilised by beta rays. Implants are packaged in a vial that, in turn, is placed inside a plastic container closed by a cap with safety seal and bearing a label with the identification data of the implant. Then the plastic container is placed inside a cardboard box bearing the same label. Further two copies of the label are into the cardboard box, to be placed on the implant passport and on the...
Open the catalog to page 19All IML UNIVERSE 2 catalogs and technical brochures
-
CATALOGUE HEX RP
52 Pages
-
CATALOGUE HEX RP
52 Pages
-
CATALOGUE CC NP
40 Pages
-
CATALOGUE CC RP
47 Pages





