
Catalog excerpts

Integra® Tissue Technologies Limit uncertainty with a leader in collagen technology
Open the catalog to page 1
Integra® Tissue Technologies Integra® Dermal Regeneration Template PriMatrix® Dermal Repair Scaffold A Pioneer in Regenerative Medicine Integra LifeSciences, a wordwide leader in medical technology, has offered innovative solutions to clinicians and patients for over 25 years. In 1996, the FDA approved the Company's first product, Integra® Dermal Regeneration Template, a collagen matrix designed as a skin replacement system for the treatment of third-degree burns. Integra® Dermal Regeneration Template was the first product approved with a claim of regeneration of dermal tissue. Advances in...
Open the catalog to page 2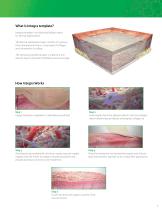
What is Integra template? Integra template is an advanced bilayer matrix for dermal regeneration. The dermal replacement layer consists of a porous, three-dimensional matrix, comprised of collagen and chondroitin-6-sulfate. The temporary epidermal layer is made of a thin silicone layer to provide immediate wound coverage. Step 1 Integra template is applied to a debrided wound bed. Step 2 Cells migrate from the adjacent dermis into the collagen matrix where they synthesize and deposit collagen to Step 3 Simultaneously, endothelial cells from nearby vascular supply migrate into the matrix to...
Open the catalog to page 3
Integra® Tissue Technologies What is PriMatrix? PriMatrix is a unique dermal repair scaffold for the management of a broad range of wound types, including diabetic and venous ulcers. Made of pure, all-natural collagen, this novel dermal matrix provides an ideal environment to support the cellular repopulation and revascularization processes critical in wound healing. How PriMatrix Works PriMatrix is cell-friendly dermal collagen and acts as a scaffold for cells and bloods vessels as the body repairs the wound. PriMatrix Collagen Scaffold PriMatrix is a dermal repair scaffold that is...
Open the catalog to page 4
Integra® Product Portfolio Integra® Dermal Regeneration Template The first FDA approved bilayer matrix for dermal regeneration in 3rd degree burns and scar contractures Integra® Meshed Dermal Regeneration Template The only FDA approved pre-meshed bilayer matrix for dermal regeneration in 3rd degree burn and scar contractures Integra® Bilayer Wound Matrix A bilayer matrix for a variety of wounds that provides a scaffold for cellular invasion and capillary growth Integra® Meshed Bilayer Wound Matrix A pre-meshed bilayer matrix for wound management that can be used in conjunction with negative...
Open the catalog to page 5
Integra® Tissue Technologies Brief Summary Consult Package Insert for Full Prescribing Information Integra® Dermal Regeneration Template Integra® Meshed Dermal Regeneration Template Description Integra Dermal Regeneration Template, available in Meshed and Non-Meshed configurations (Integra template) is a bilayer membrane system for skin replacement. The dermal replacement layer is made of a porous matrix of fibers of cross-linked bovine tendon collagen and glycosaminoglycan (chondroitin-6-sulfate) that is manufactured with a controlled porosity and defined degradation rate. The epidermal...
Open the catalog to page 6
Brief Summary Consult Package Insert for Full Prescribing Information Integra® Wound Matrix Indications Integra® Matrix Wound Dressing is indicated for the management of wounds including: partial and full-thickness wounds, pressure ulcers, venous ulcers, diabetic ulcers, chronic vascular ulcers, tunneled/undermined wounds, surgical wounds (donor sites/grafts, post-Moh's surgery, post-laser surgery, podiatric, wound dehiscence), trauma wounds (abrasions, lacerations, second-degree burns, skin tears) and draining wounds. The device is intended for one-time use. Contraindicatons • This device...
Open the catalog to page 7
Ordering Information Integra Dermal Regeneration Template For more information or to place an order, please contact: United States, Canada, Asia, Pacific, Latin America USA 800-654-2873 ■ 888-980-7742 fax International +1 609-936-5400 ■ +1 609-750-4259 fax integralife.com/contact Integra LifeSciences Corporation 109 Morgan Lane Plainsboro, NJ 08536 ■ USA TEI Biosciences, a subsidiary of Integra LifeSciences Corporation 7 Elkins Street Boston, MA 02127 ■ USA PriMatrix, Integra and the Integra logo are registered trademarks of Integra LifeSciences Corporation or its subsidiaries in the United...
Open the catalog to page 8All Integra LifeSciences catalogs and technical brochures
-
capture high-torque surgical
12 Pages
-
cadence
4 Pages
-
DuraGen® Secure
8 Pages
-
transducer
2 Pages
-
MAYFIELD®
12 Pages
-
SurgiMend® Hernia Brochure
8 Pages
-
MediHoney® Brochure
9 Pages
-
AmnioExcel® OR Sell Sheet
2 Pages
-
PyroCarbon Implants
8 Pages
-
Wrist Solutions
6 Pages
-
Lower Extremity Reconstruction
28 Pages
-
Integra® Lighting Solutions
4 Pages
-
Integra® CUSA® Excel+
1 Pages
-
CUSA® Excel+ System
4 Pages
-
DuraGen® matrix
2 Pages
-
SurgiMend®
6 Pages
-
DIABETIC SKIN ULCER
16 Pages
-
Auragen™ Cortical Electrodes
4 Pages
-
DuraGen XS
2 Pages
-
Suturable DuraGen™
4 Pages
-
DuraGen Plus
4 Pages
-
MAYFIELD® 2
3 Pages
-
Hermetic Plus™
2 Pages
-
AccuDrain™
4 Pages
-
Oral Surgery
107 Pages
-
Catalog - Endodontics
110 Pages
-
Catalog - Cassette
36 Pages
-
Brochure - Liberator
6 Pages
-
Catalog - Precision Dental
28 Pages
-
LICOX
4 Pages
-
Integra® Licox®
2 Pages
-
Integra® Camino® Flex
6 Pages
-
Camino
10 Pages
-
Jarit Sterilization System
11 Pages
-
Pain Management Procedure Trays
26 Pages
-
CUSA Excel+
8 Pages
-
Integra Miltex
663 Pages
-
Integra VATS
28 Pages
-
Cardiovascular
285 Pages
-
Padgett
322 Pages
-
Integra Podiatry
130 Pages
-
Integra Jarit
916 Pages
-
Integra Laparoscopic
90 Pages
-
Extremity-Reconstructive Surgery
87 Pages
-
Integra Ruggles Redmond
242 Pages
-
Stereotaxy
28 Pages
-
CUSA NXT/Selector
8 Pages
-
Neuro Operating Room
177 Pages
-
CUSA EXcel
12 Pages
-
Neuromonitoring
76 Pages














































































