
Catalog excerpts

The ultimate imaging the ultimate imaging software suite software suite for for clinical research clinical research
Open the catalog to page 1
The Myrian® suite is the first customizable solution for the quantification and follow-up of multimodality and multiparametric imaging endpoints in oncology and chronic diseases. Main TherapeuTiC areas Oncology Neurology Cardiovascular Respiratory Musculoskeletal CliniCal MOdules Myrian® XL-Onco Myrian® XP-Liver Myrian® XP-Breast Myrian® XP-Prostate Myrian® XP-FemalePelvis Myrian® XP-Colon Myrian® XP-LungNodule Myrian® XP-Lung Myrian® XP-AbdoFat Myrian® XT-Brain Myrian® XT-Cardiac Myrian® XP-Vessel Myrian® XP-Ortho Main iMaGinG MOdaliTies CT MRI X-Ray PET PET/CT SPECT SPECT/CT Pathology...
Open the catalog to page 2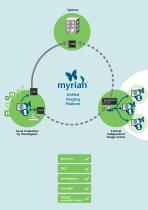
Unified Imaging Platform Local evaluation by investigator Central independent image review Sponsors CRO Investigators Core labs Clinical research centers
Open the catalog to page 3
Main features Learn more about Myrian® suite for clinical research in 8 points. Myrian® complete set of clinical applications provides standard and accurate imaging endpoints in a wide range of therapeutic areas, whatever the modality used. Automation, performed at different levels of the application, allows for more reproducible measurements and time savings. With a full implementation of guidelines such as RECIST, objective evaluation of response to treatment is easier to achieve. Myrian® offers an intuitive and user-centric multi-language interface which allows a short learning curve as...
Open the catalog to page 4
standard and accurate imaging endpoints Objective evaluation of response to treatment early detection of treatment failure Faster time-to-market ability to implement new imaging biomarkers CrO efficient workflow and time saving standard and accurate imaging endpoints smooth integration remote reading capabilities Flexible and customizable solution ability to implement new imaging biomarkers smooth integration with hospital iT environment ease-of-use efficient workflow and time saving Compliance with guidelines such as reCisT unified solution for clinical research and clinical routine CTMs /...
Open the catalog to page 5
Offices Amsterdam Berlin Istanbul Kuala Lumpur Montpellier Moscow New York Paris São Paulo Shanghai Tokyo Myrian® software is developed, distributed and manufactured by Intrasense SA. Intrasense® and Myrian® are either registered trademarks, or trademarks of Intrasense SA in France and/or other countries. Copyright Intrasense – 1231, avenue du Mondial 98, 34000 Montpellier (France) – Tel. +33 467 130 130. All rights reserved. All other product names, company names, brand names, trademarks and logos are the property of their respective owners. Edition date: 05/2014....
Open the catalog to page 6All Intrasense catalogs and technical brochures
-
Myrian XT-Brain
2 Pages
-
Myrian XP-Vessel
2 Pages
-
Myrian XP-Prostate
2 Pages
-
Myrian XP-LungNodule
2 Pages
-
Myrian XP-Lung
2 Pages
-
Myrian XP-Liver
2 Pages
-
Myrian XP-FemalePelvis
2 Pages
-
Myrian XP-Colon
2 Pages
-
Myrian XP-Breast
2 Pages
-
myrian for MRI
2 Pages
-
myrian Application server
6 Pages
-
Myrian XL-Onco
2 Pages
-
Myrian Expert VL
2 Pages
-
Myrian Expert
2 Pages
-
Myrian Advance
2 Pages
-
Myrian Pro
2 Pages

















