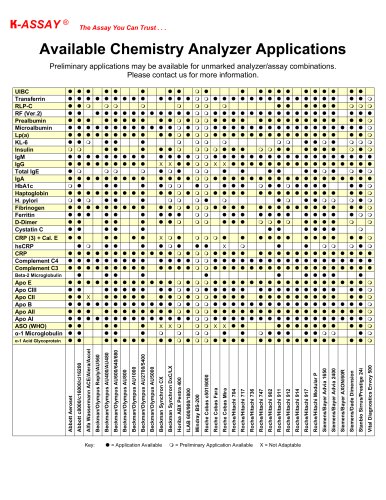
Catalog excerpts

Synonyms: Rheumatoid Factor (Version 2), R-1 Intended Use: For the quantitative determination of human IgG rheumatoid factor antibodies in patient serum or plasma (citric acid, EDTA, or lithium heparin) based on immunoturbidimetric in vitro assay. Manufacturer: KAMIYA BIOMEDICAL COMPANY Website: www.k-assay.com Address: 12779 Gateway Drive KAMIYA BIOMEDICAL COMPANY SDS for KAI-230 (R-2) Rev. 2020-09-08 Page 1 of 4
Open the catalog to page 1
6. ACCIDENTAL RELEASE MEASURES
Open the catalog to page 2
This product is a mixture that contains a very low concentration of the following substance. Here are details for the substance in pure form. Regulatory information with regard to this preparation in your country or region should be examined on your own responsibility. KAMIYA BIOMEDICAL COMPANY SDS for KAI-230 (R-2) Rev. 2020-09-08 Page 3 of 4
Open the catalog to page 3
16. OTHER INFORMATION / DISCLAIMER This product is for in vitro use only. It is not to be used internally in humans or animals. The information, data, and recommendations contained herein are based upon information believed by KAMIYA BIOMEDICAL COMPANY (KBC) to be accurate, but does not purport to be all-inclusive and shall be used only as a guide. KBC neither warrants the accuracy of this information nor assumes any legal responsibility in connection with its dissemination. KBC shall not be held liable for any damage resulting from handling or from contact with the above product. It is the...
Open the catalog to page 4All Kamiya Biomedical Company LLC catalogs and technical brochures
-
Assay Flyer
4 Pages