Catalog excerpts

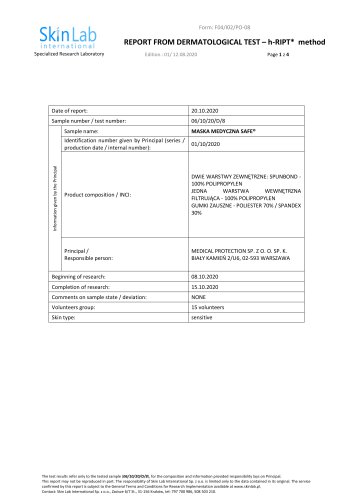
REPORT Contact person RISE Josefin Seth Caous Chemistry, Biomaterials and Textiles +46 10 516 59 96 josefin.caous@ri.se RISE ID 111-2 Svea smittskydd & beredskap AB Att. Frank Albazi BJÖRNSTIGEN 12 17072 Solna Bacterial filtration efficiency of medical face mask (1 appendix) Assignment Evaluate bacterial filtration efficiency (BFE) of a medical face mask following the guideline of SS-EN 14683:2019 section 5.2.2. Test item Test item delivered to RISE: 1. Medical Mask SAFE® Type IIR / ES Figure 1. Package of the test item Methods While wearing gloves, the test item was placed in a humid chamber and conditioned overnight at 85% ± 5 relative humidity, for minimum four hours. Bacterial suspension of Staphylococcus aureus (ATCC 6538) with a concentration of 1 x 104 colony forming units (CFU) per mL was prepared in peptone water. The inoculum was serially diluted and plated on tryptic soy agar (TSA) for CFU count. A 10 ml syringe was filled with the bacterial suspension and mounted to a nebulizer, adjusted to deliver one mL suspension in one minute. Aerosols were formed by applying air pressure of 35 kPa to the specified volume of bacterial suspension delivered into the aerosol chamber by the nebulizer at a controlled speed. The aerosols were drawn through a six stage impactor by the means of a vacuum pump. At each of the six stages in the impactor a TSA agar plate was mounted, collecting the aerosols of defined size at each stage, according to table 1. Aerosols were produced for one minute, followed by one minute air, for each sample except the negative control were two minutes air was applied, without bacterial aerosols. A minimum of RISE Research Institutes of Sweden AB Postal address Office location Arvid Wallgrens Backe 20 SE-413 46 GÖTEBORG Sweden Arvid Wallgrens Backe 20 Vån 5 SE-413 46 GÖTEBORG This document may not be reproduced other than in full, except with the prior written approval of RI
Open the catalog to page 1
two positive controls (without face mask) were sampled, one prior to the tested face masks and one after. The negative control, without bacterial suspension or face mask, was sampled last. The face masks, one at a time, was mounted as shown in figure 1, with the inside facing the aerosol challenge. For each of the five tested face masks, as well as controls, aerosols were collected on six agar plates. The ends of the face masks were cut off in order to mount them flat in the impactor. A circular area with a diameter of 8 cm was tested for each face mask, resulting in an area of ~50 cm2. The...
Open the catalog to page 2
Table 2. BFE for each test specimen Medical Mask SAFE® Type IIR / ES 1 2 3 4 5 Conclusion The test item, “Medical Mask SAFE® Type IIR / ES” fulfilled the requirement of Type II masks, with a filtration efficiency above 98% for all five samples. RISE Research Institutes of Sweden AB Chemistry and Materials - Medical Device Technology Josefin Seth Caous Sara Bogren References 1. SS-EN 14683:2019+AC:2019 Medical face masks – Requirements and test methods 2. Andersen, Ariel A. "NEW SAMPLER FOR THE COLLECTION, SIZING, AND ENUMERATION OF VIABLE AIRBORNE PARTICLES." The Journal of Bacteriology...
Open the catalog to page 3
Figure A1: Set-up with a test item mounted in the impactor. Figure A2: Typical CFU distribution on agar plates from positive controls RISE Research Institutes of Sweden AB
Open the catalog to page 4
Figure A3: Typical CFU distribution on agar plates were the aerosols have been filtered through face mask 5 of 5 from product “Medical Mask SAFE® Type IIR / ES”. RISE Research Institutes of Sweden
Open the catalog to page 5