
Catalog excerpts

OsteoBridge™ IDSF Intramedullary Diaphyseal Segmental Fixation Surgical Technique This surgical technique applies only to the U.S.
Open the catalog to page 1
Caution Federal law restricts this device to sale by or on the order of a physician. Caution The following product descriptions contain detailed information on the recommended procedure (and associated surgical techniques) for Merete® implants and instruments. Training in the correct handling of implants and instruments is only to be executed by an authorized Merete representative.
Open the catalog to page 2
Table of Contents 1. Description 4 1.1. Indications 1.2. Contraindications 5 1.3. MRI Safety Information 6 2. General Information 6 3. System Compatibility 7 4. Surgical Technique 13 4.1. Preoperative Planning 14 4.2. Preparing the Defect to be Treated
Open the catalog to page 3
OsteoBridge™ Intramedullary Diaphyseal Segmental Fixation System Description 1. Description Warning Use of implants contrary to intended purpose. Risk of injury due to implant failure. Implant must only be used in accordance with intended purpose. The OsteoBridge™ Intramedullary Diaphyseal Segmental Defect Fixation System (IDSF) is intended to be used in the management of segmental diaphyseal bone loss secondary to radical bone loss and/or resection due to tumors in either humerus, tibia or femur in oncology patients. The device is divided into three categories/product size ranges that are...
Open the catalog to page 4
OsteoBridge™ Intramedullary Diaphyseal Segmental Fixation System Description 1.1. Indications for Use • Long-term stabilization of major bone defects resulting from • Radical bone loss due to tumors and/or metastases Bone resections following tumors and/or metastases • For use only in the diaphyseal region of humerus, tibia and femur The OsteoBridge ™ Intramedullary Diaphyseal Segmental Defect Fixation System (IDSF) may be inserted non-cemented or cemented (PMMA) according to the specifications supplied by the cement manufacturer. Do not additionally lock the nail after cementing. 1.2....
Open the catalog to page 5
OsteoBridge™ Intramedullary Diaphyseal Segmental Fixation System Description/MRI Safety Information 1.3. MRI Safety Information MRI Safety Information/Indications for Use Non-clinical testing has demonstrated that the Merete OsteoBridge™ Intramedullary Diaphyseal Segmental Defect Fixation Implant System (IDSF) (consisting of two cemented or non-cemented intramedullary stems and one or two spacers made of TiAl6V4 ELI (ISO 5832-3)) is MR conditional. A patient with the entire assembled Merete OsteoBridge™ Intramedullary Diaphyseal Segmental Defect Fixation Implant System (IDSF) can be safely...
Open the catalog to page 6
OsteoBridge™ Intramedullary Diaphyseal Segmental Fixation System General Information/System Compatibility Combination with products from other manufacturers. Risk of injury due to implant failure! Do not combine the implant components with products from other manufacturers. Use of implants which have been previously used. Risk of injury due to premature implant failure! Risk of sepsis! Implants are only approved for single use, not reuse. Use of contaminated implants/instruments. Risk of Sepsis! Use only implants/instruments without visible contamination. Risk of infection due to...
Open the catalog to page 7
OsteoBridge™ Intramedullary Diaphyseal Segmental Fixation System System Compatibility NOTE - umerus Spacer, 20 mm outer diameter and 10 mm H inner diameter Spacer Connector for Spacer Nail Diameter (Connection DIA. 10 mm) Diameter Caution If locking is required: Use 3.8 mm interlocking screws (GB33818S-GB33832S) with 7 mm and 8 mm diameter nails ONLY. Use 5 mm interlocking screws (GB35020S-GB35065S) with 9 mm and 10 mm diameter nails. Merete Tech
Open the catalog to page 8
OsteoBridge™ Intramedullary Diaphyseal Segmental Fixation System System Compatibility NOTE - ibia Spacer, 25 mm outer diameter and 14 mm inner T diameter Spacer Connector for Spacer Nail Diameter (Connection DIA. 14 mm) Diameter NOTE If locking is required: Use 5 mm interlocking screws (GB
Open the catalog to page 9
OsteoBridge™ Intramedullary Diaphyseal Segmental Fixation System System Compatibility NOTE - emur Spacer, 34 mm outer diameter and 16 mm F inner diameter Spacer Connector for Spacer Nail Diameter (Connection DIA. 16 mm) Diameter Length NOTE If locking is required: Use 5 mm interlocking screws (GB35020S-GB35065S). Merete Technologies
Open the catalog to page 10
4.1. Preoperative Planning 12 4.2. Preparing the Defect to be Treated 14 4.2.1. Non-Cemented Implantation 15 4.2.2. Cemented Implantation 16 4.3. Implant Selection
Open the catalog to page 13
OsteoBridge™ Intramedullary Diaphyseal Segmental Fixation System Surgical Technique 4.1. Preoperative Planning The surgical technique shown here serves as an example to help illustrate the basic procedure for implanting the OsteoBridge™IDSF System. Merete GmbH, manufacturer of this medical product, does not stipulate that this or any other treatment method is to be used for any specific patient. The responsibility for selecting a suitable method of treating a patient lies with that patient’s operating physician. Patient information is to be provided in accordance with the product...
Open the catalog to page 14
OsteoBridge™ Intramedullary Diaphyseal Segmental Fixation System Surgical Technique Figure 3: X-ray Templates
Open the catalog to page 15
OsteoBridge™ Intramedullary Diaphyseal Segmental Fixation System Surgical Technique Please note the following when using the instruments: Use the Ratcheting Screwdriver (Ref. GB90213) with 3.8 mm and 5.0 mm diameter interlocking screws for screw placement in bone and nail. Screwdriver for Interlocking Screws Nails: 7 mm and 8 mm diameter Interlocking screws: 3.8 mm diameter (Ref. GB33818S - GB33832S) Ref. GB90209 Hex 2.5 mm Screwdriver for 3.8 mm Interlocking Screws Ref. GB90204 Hex 3.5 mm Screwdriver for 5.0 mm Interlocking Screws Screwdriver for Clamping Screws Spacer: 20 and 25 mm...
Open the catalog to page 16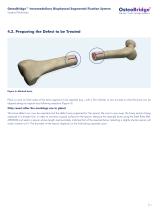
OsteoBridge™ Intramedullary Diaphyseal Segmental Fixation System Surgical Technique 4.2. Preparing the Defect to be Treated Figure 4: Marked bone. Place a mark on both sides of the bone segment to be resected (e.g., with a Skin Marker or two K-wires) so that the bone can be aligned along its original axis following resection (Figure 4). Only resect after the markings are in place! The bone defect can now be resected and the defect zone prepared for the spacer. Be sure to saw away the bone section being replaced in a straight line, in order to maintain a good surface for the spacer. Measure...
Open the catalog to page 17All Merete catalogs and technical brochures
-
BioBall System Catalogue
24 Pages
-
MetaCun II and Duo MetaCun II
16 Pages
-
SCARFixTM
12 Pages
-
Merete Cannulated HCS
2 Pages






