Catalog excerpts

Quick Reference Echo Screening Indication: The MitraClip Clip Delivery System is indicated for the percutaneous reduction of significant symptomatic mitral regurgitation (MR ≥ 3+) due to primary abnormality of the mitral apparatus [degenerative MR] in patients who have been determined to be at prohibitive risk for mitral valve surgery by a heart team, which includes a cardiac surgeon experienced in mitral valve surgery and a cardiologist experienced in mitral valve disease, and in whom existing comorbidities would not preclude the expected benefit from reduction of the mitral regurgitation. See Important Safety Information Referenced Within.
Open the catalog to page 1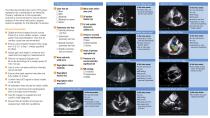
Parasternal Short Axis View: Aortic Valve Level The following transthoracic echo (TTE) views represent key considerations for MitraClip Therapy. Adherence to this systematic protocol is recommended to ensure efficient analysis of the mitral valve and to assess anatomic eligibility for the MitraClip Procedure. General Comments ■ Digital archived images should include three (3) or more cardiac cycles—unless patient has atrial fibrillation, then five (5) cardiac cycles are recommended ■ Ensure color Doppler Nyquist limits range from 0.5-0.7 m/sec—unless specified for PISA ■ Adjust gain and...
Open the catalog to page 2
MitrAClip Clip DeliVery SySteM iNDiCAtiON FOr USe The MitraClip Clip Delivery System is indicated for the percutaneous reduction of significant symptomatic mitral regurgitation (MR ≥ 3+) due to primary abnormality of the mitral apparatus [degenerative MR] in patients who have been determined to be at prohibitive risk for mitral valve surgery by a heart team, which includes a cardiac surgeon experienced in mitral valve surgery and a cardiologist experienced in mitral valve disease, and in whom existing comorbidities would not preclude the expected benefit from reduction of the mitral...
Open the catalog to page 3
• The inside of the outer pouch is not a sterile barrier. The inner pouch within the outer pouch is the sterile barrier. Only the contents of the inner pouch should be considered sterile. The outside surface of the inner pouch is NOT sterile. • Note the “Use by” date specified on the package. pOteNtiAl COMpliCAtiONS AND ADVerSe eVeNtS The following ANTICIPATED EVENTS have been identified as possible complications of the MitraClip procedure. Allergic reaction (anesthetic, contrast, Heparin, nickel alloy, latex); Aneurysm or pseudo-aneurysm; Arrhythmias; Atrial fibrillation; Atrial septal...
Open the catalog to page 4
Abbott Vascular 4045 Campbell Avenue, Menlo Park, CA 94025 USA, Tel: 1.650.833.1600 MitraClip is a trademark of the Abbott Group of Companies. www.AbbottVascular.com PML04106 Rev. A © 2013 Abbott. All rights reserved. Abbott Vascular
Open the catalog to page 5