Catalog excerpts
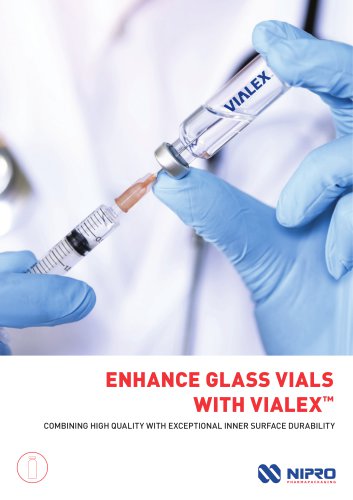
ENHANCE GLASS VIALS WITH VIALEX™ COMBINING HIGH QUALITY WITH EXCEPTIONAL INNER SURFACE DURABILITY
Open the catalog to page 1
ENHANCE Vials with VIALEX™ The past few years have seen a significant acceleration in the development of complex drugs for injection. In fact, more than 80% of drugs for injection in the current drug pipeline are large molecules, including biologics, oligonucleotides, and gene therapy drugs. Given their high value and sensitivity to drugcontainer interactions, selecting the right primary container for these products is critical. Proper selection is essential as it contributes to stable fill-finish operations, as well as maintains the stability, efficacy, and safety of the drug product....
Open the catalog to page 2
Optimized filling-line performance ENHANCE glass vials are very consistent in size and shape. Each vial is controlled carefully by camera inspection 100% and in-line for a broad array of dimensional parameters. The precise dimensions support a reliable handling during the fill-finish process. Crafted from top-of-the-line type I, borosilicate glass tubing, ENHANCE vials offer an extremely consistent wall thickness. It goes hand in hand with reduced glass-to-glass contact throughout the entire vial production process and leads to an increased mechanical durability. The in-depth, 100% in-line...
Open the catalog to page 3
Outstanding Drug-Container Compatibility INNOVATIVE TECHNOLOGY THAT YIELDS VIALS WITH EXCEPTIONAL INNER SURFACE DURABILITY Less interactions of drug molecules and formulations with the inner glass surface Water for injection | Aqueous NaCl solution • Optimized lyophilization process (less fogging) • • Reduced risk of glass delamination Reduced risk of glass delamination Superior to Current Alternatives VIALEX Process Control Sodium deposits removed Inner glass surface improved Additional material (risk factor) Extensive regulatory work required Glass chemistry Change Inner Surface Coating
Open the catalog to page 4
VIALEX Manufacturing Process And Benefits The inner glass surface of the vial is treated with the Nipro proprietary PICVA¹ process. [no extra materials, no change in glass chemistry, 100% in-line inspected]. It mitigates the effects from the converting process twofold. Sodium concentration reduced Inner glass surface improved Low levels of extractables & leachables | Reduced alkalinity on the glass surface | Enhanced chemical durability Less interactions of drug molecules and formulations with the inner glass surface | Lower pH shift | Reduced risk of glass delamination | Optimized...
Open the catalog to page 5
VIALEX™-TREATED VIALS : TESTS AND RESULTS Vialex-treated vials were tested according to industry standards under challenging conditions and with aggressive buffer solutions. The results underline the exceptional inner surface durability when compared to industry standard vials (Ind. Std. Vial) VIALEX-treated vials reveal an unprecedented surface hydrolytic resistance with a surface durability like original glass tubing. They consistently perform ≤25% of the max. limit. Enhanced Chemical Durability L O N G -T E R M S TA B I L I T Y S T U D Y SEM: 24 weeks at 40°C – 51exp | Phosphate, High...
Open the catalog to page 6
SEM: 24 weeks at 40°C – 51exp | Phosphate, High Purity Water, Citrate, NaCl with TS In terms of extractables, VIALEX-treated vials reveal a lower level of extractables when compared to Ind. Std. Vials. Fill time with high purity water (weeks) VIALEX Ind. Std. Vial Fill time with citrate (weeks) VIALEX Ind. Std. Vial Fill time with phosphate (weeks) VIALEX Ind. Std. Vial Fill time with 0.9% NaCl with terminal sterilization (weeks) Reduced pH shift NACL 0.9%: PH AT START 5.2 - WITH TS - AT 121°C – 2 ML VIAL VIALEX-treated vials exhibit the lowest level of pH shift. While ammonium...
Open the catalog to page 7
Nipro PharmaPackaging is specialized in developing and manufacturing advanced pharma packaging products and complete packaging solutions for early development drugs or the enhancement of packaging solutions for existing drugs. With a worldwide manufacturing footprint of 19 plants, multiple sales offices, and internal lab services, Nipro PharmaPackaging offers an exceptional service platform. Through our personnel, products, and services, Nipro PharmaPackaging enables you to provide a safer and healthier administration to your customers. Nipro PharmaPackaging is part of Nipro Corporation...
Open the catalog to page 8All Nipro Middle East FZE catalogs and technical brochures
-
VASCULAR ACCEESS
36 Pages
-
MINI DIALYZER WORKSHOP
4 Pages
-
GRAVINEX
6 Pages
-
CL Fine
2 Pages
-
CL-FIne
8 Pages
-
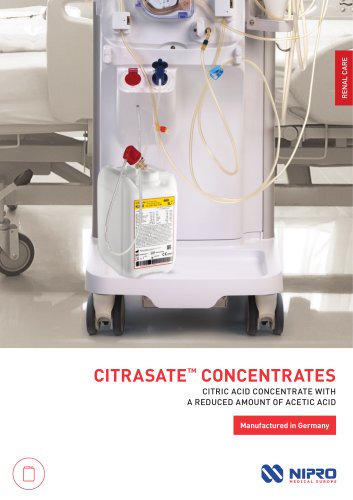
CITRASATE™ CONCENTRATES
6 Pages
-
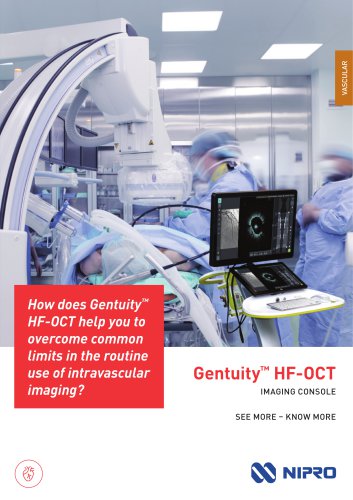
Gentuity™ HF-OCT
4 Pages
-
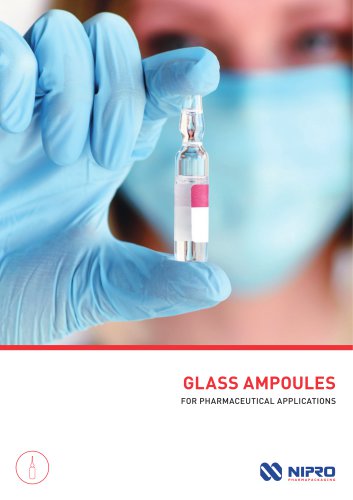
GLASS AMPOULES
8 Pages
-
SUREFUSER+ Brochure
6 Pages
-
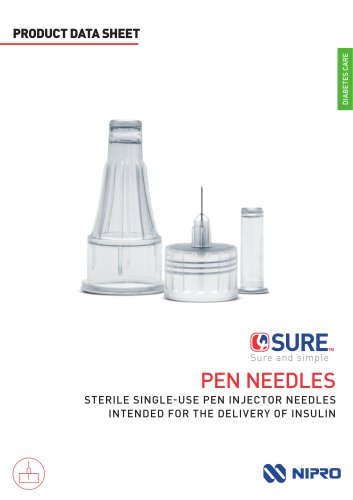
4SURE Pen Needles Datasheet
4 Pages
-
4SURE Pen Needles Brochure
2 Pages