Catalog excerpts

FLOWOX™ Enhancement of blood flow to lower limbs [EN] INSTRUCTION MANUAL | Page 3 [IT] MANUALE D’USO | Page 47 [NO] INSTRUKSJONER FOR BRUK | Page 17 [FR] MODE D’EMPLOI | Page 63 610-00013 FLOWOX™ 2,0 Instructions for Use (IFU) Manual Rev 1
Open the catalog to page 1
610-00013 FLOWOX™ 2,0 Instructions for Use (IFU) Manual Rev 17
Open the catalog to page 2
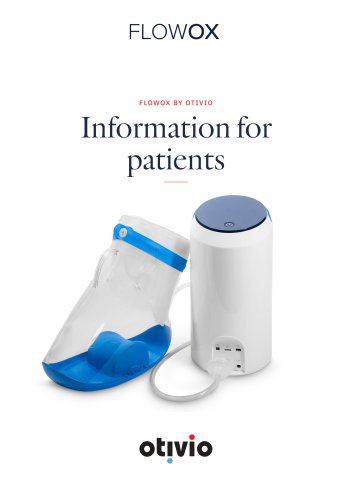
INSTRUCTION MANUAL [EN] WELCOME FlowOx™ is a medical device in accordance with the Medical Device Directive 93/42/EEC, which improves blood flow to your lower limb. The patented technology is based on several years of research and development. Please carefully read this instruction manual before using the device. The Quick Guide [0] should be kept handy. Otivio and its distributors decline any responsibility for any use of FlowOx™ that does not comply with this instruction manual. HOW IT WORKS The user puts the foot and leg into a Pressure Chamber [1] which is connected to the Control Unit...
Open the catalog to page 3
INITIAL SETUP OF FLOWOX™ BY CLINICIAN Please read this FlowOx™ instruction manual and refer to the drawings at the beginning of the instruction manual. (A) Unpack and verify that all items are provided and free from damage (see overview picture). If any parts are missing or damaged, please contact your clinician or distributor. The Control Unit box contains the Quick Guide [0], Instructions for use (IFU) Manual [0], the Control Unit [4], the power supply [2], Timecard [5] (if supplied), USB drive with FlowOx™ Software [32] data transfer cable [11]. The Pressure Chamber box contains the...
Open the catalog to page 4
(B) Fitting FlowOx™. (a) Check that the Pressure Chamber [1] fits the patient and is comfortable. Only the arch of the foot should rest lightly on the top of the Positioner [16]. Note: The foot should not touch the bottom of the Pressure Chamber [1]. The Positioner [16] is not a weight bearing support device. (b) The Seal [20] should be comfortable on the bare calf. (c) Ask the patient to put their Pressure Chamber [1] on and take it off several times. Check that the patient can do this without harming the skin or disrupting any wound dressings. Note: It is recommended that a non-shedding...
Open the catalog to page 5
VIEWING TREATMENT DATA (CLINICIAN) Note: Connecting a PC to the Control Unit may only be done by a trained professional. PC must only be connected to the Control Unit outside of the patient area. Before connecting the Control Unit to a PC with the USB, make sure the PC has the latest security updates and an up-to-date antivirus software installed. Installing FlowOx™ Software: FlowOx™ Software enables the clinician to view the treatment data, get or delete treatment data from the Control Unit [4], synchronize the Control Unit [4] time with the local time on the PC, and view the remaining...
Open the catalog to page 6
● The inside of the Pressure Chamber [1] needs to be visually inspected both prior to and after use for any contamination such as wound exudate, dirt, and fibres ● Clean the Pressure Chamber [1], Padding [17], Seal [20], and Positioner [16] in accordance with section “Cleaning”, if visible contamination is present ● Check that the Hose with Filter [24] is visibly clean. Do not use the Pressure Chamber [1], if the Hose with Filter [24] contains any blood or liquid, in this case discard the Pressure Chamber [1] and use a new one ● Care should be taken when putting on and taking off the...
Open the catalog to page 7
COMPLAINTS AND ADVERSE EVENTS Complaints about FlowOx™, including any adverse events experienced in its use, should be commiunicated to your distributor or directly to Otivio at info@otivio.com. Serious adverse events should also be reported directly to Otivio at info@otivio.com and the relevant authorities where you live, contacts are shown below: Belgium: vigilance.meddev@fagg-afmps.be Denmark: med-udstyr@dkma.dk Germany: medizinprodukte@bfarm.de Ireland: devicesafety@hpra.ie Italy: dgfdm@postacert.sanita.it Spain: psvigilancia@aemps.es France: medicaldevicesvigilance@ansm.sante.fr...
Open the catalog to page 8
Environmental and handling conditions: Operation Temperature range: +15 to +40°C. Note: if device is taken from storage that is outside of operating temperature (15 – 40°C), wait at least 2 hours before using the device. ● Operation Air Humidity: 15 – 95 % ● Operation Ambient pressure: 70 – 106 kPa (525 – 795 mmHg) ● Storage and transport Temperature range: - 25 to +70 °C ● Storage and transport Air Humidity: Up to 93 % relative humidity at +70 °C ● Storage and transport Ambient pressure: 50 – 106 kPa ● Degree of enclosure protection: IP21 (Protected against solid objects over 12mm e.g.,...
Open the catalog to page 9
BRUKSANVISNING [SV] VÄLKOMMEN FlowOx™ är en medicinteknisk produkt som följer direktivet 93/42/EEG för medicintekniska produkter och som förbättrar blodflödet till dina nedre lemmar. Den patenterade tekniken är baserad på flera års forskning och utveckling. Läs noga igenom denna bruksanvisning innan enheten används. Snabbguiden [0] bör hållas nära till hands. Otivio och företagets distributörer ansvarar inte för någon användning av FlowOx™ som inte följer denna bruksanvisning. HUR DEN FUNGERAR Användaren sätter foten och benet in i en tryckkammare [1] som är ansluten till kontrollenheten...
Open the catalog to page 10
INITIAL INSTÄLLNING AV FLOWOX™ AV LÄKARE Läs denna bruksanvisning för FlowOx™ och se ritningarna i början av bruksanvisningen. (A) Packa upp och kontrollera att alla delar finns med och är oskadda (se översiktsbilden). Kontakta din läkare eller distributör om delar saknas eller är skadade. Kontrollenhetens låda innehåller snabbguiden [0], bruksanvisningen [0], kontrollenheten [4], strömförsörjningen [2], tidkortet [5] (om det medföljer), USB-enheten med FlowOx™-programvara [32] och dataöverföringskabeln [11]. Tryckkammarens låda innehåller snabbguiden [0], bruksanvisningen [0],...
Open the catalog to page 11All Otivio catalogs and technical brochures
-
FlowOx™ 2.0 Quick Guide
22 Pages
-
Information for patients
6 Pages
-
General overview
2 Pages