
Catalog excerpts

SD BIOSENSOR PRODUCT CATALOG POC in-vitro Total Platform Company Global leader and a Korean pioneer to provide full-line solution from initial screening to confirmatory tests,
Open the catalog to page 1
Products Beginning of all things that protect lives SD Biosensor, Inc., with its slogan ‘Beginning of all things that protect CATALOG lives,’ global in-vitro diagnostics 2022 is a everyone’s quality of life bycompany that contributes to improving diagnosing diseases quickly and accurately. SD Biosensor is a Total Solution Provider in the IVD industry that develops and researches innovative diagnostic platforms. Continuous technological innovations for a global biotech company Based on R&D know-how, Mass Production Capacity, and Global Sales Network, SD Biosensor, Inc. will continue to grow...
Open the catalog to page 2
Screening Test Confirmatory Test 01 Self test, qualitative test that can be tested by patient like a COVID-19 home test kit (for screening purpose) ■ BGMS ■ STANDARD Q Tests that can be examined by a medical staff Qualitative/Quantitative test ■ BGMS ■ STANDARD Q Test to diagnose the presence or absence of disease through medical staff ■ BGMS ■ STANDARD Q ■ STANDARD F ■ STANDARD M
Open the catalog to page 3
SD BIOSENSOR HISTORY OF INNOVATION Since 2010, SD BIOSENSOR has grown and evolved to make the world healthier through our innovative IVD products. Our goal is to be the global leading in-vitro diagnostics company. From starting with BGMS products, we have expanded our business to STANDARD Q(RDT), STANDARD F(FIA), STANDARD E(ELISA) and STANDARD M(POC Molecular Diagnostic Platform). We are never complacent where we are but strive to become the No.1 global in-vitro diagnostics company through continuous technological innovations. • Established SD BIOSENSOR, Inc. • Obtained FDA Approval for SD...
Open the catalog to page 4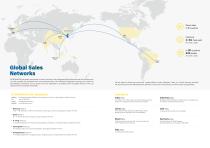
Direct sales in 7 countries Office Global Sales Networks SD BIOSENSOR has grown and evolved in chronic care and in-vitro diagnostics(IVD) industries over the last few years. Our IVD portfolio has expanded from immuno-based IVD to POC Molecular Diagnostics through the continuous technological innovations. As our product line has expanded in accordance with the global needs for IVD, our customers have increased world-wide. We are based in South Korea and have 7 global offices in India, Indonesia, China, U.S., Brazil, Germany and Italy. We also have more than 800 distribution partners in more...
Open the catalog to page 5
01 STANDARD M Molecular diagnostics STANDARD F Fluorescent immunoassay 03 STANDARD Q Rapid diagnostic test 04 STANDARD E Enzyme-Linked Immunosorbent Assay Assay Menu Respiratory Infections 14 qPCR Reagent Respiratory Infections 19 Vector Borne Disease 36 Blood Borne Disease 44 Vector Borne Disease 67 Blood Borne Disease 72 Vector Borne Disease 80 05 Chronic Care Blood Glucose Monitoring System & Chronic Care Analyzers Chronic Care - Introduction 86 Chronic Care - Technology 87
Open the catalog to page 6
STANDARD M Molecular Diagnostics STANDARD™ M is a molecular diagnostic brand including STANDARD M10, a point-of-care molecular diagnostic system, PCR reagents and other related products. STANDARD™ M10 is a versatile POC system designed for more accurate, simpler and faster clinical decision making near-the-patient using real-time PCR or real-time LAMP. STANDARD™ M10 is an automated system that integrates extraction and amplification of nucleic acids from various specimens and detection of target sequences. STANDARD™ M10 consists of STANDARD™ M10 Module and STANDARD™ M10 Console. The entire...
Open the catalog to page 7
STANDARD M10 Versatile Point-of-Care Molecular Diagnostic Platform POC Molecular Diagnostic Platform designed for more accurate, simpler and faster clinical decision making near-the-patient. Versatile Point-of-Care Molecular Diagnostic Platform FEATURES • User friendly GUI with animated guide • Seamless connectivity with HIS/LIS • Memory up to 5,000 with Ct values & amplification curves • 10.1" touch screen • Customized configuration up to 8 modules • Alternative operating system to PC (Window) • Minimized maintenance requirements • Intuitive status indicator • Small footprint...
Open the catalog to page 8
D s Multiplex real-time RT-PCR test intended for use with STANDARD™ M10 system for the s qualitative detection of nucleic acid from the SARS-CoV-2 ORF1ab(RdRp) gene and E gene in upper respiratory specimens(such as nasopharyngeal) collected from individuals suspected of COVID-19. Test type Professional Use Only c Specimen type Nasopharyngeal swab Reference Clinical Sensitivity Clinical Specificity Limit of Detection (LoD) STANDARD™ M10 Flu/RSV/SARS-CoV-2 is a multiplex real-time RT-PCR test intended for use with STANDARD M10 system for the qualitative detection of Influenza A, Influenza B,...
Open the catalog to page 9
STANDARD™ M10 MDR-TB is a multiplex real-time PCR test intended for use with STANDARD M10 system for the qualitative detection of Mycobacterium tuberculosis nucleic acids and drug-resistance against rifampicin(RIF) and isoniazid (INH) in human normal sputum or sputum sediment sample. Test type Specimen type Storage condition Advantage Professional Use Only Pretreated normal sputum, sputum sediment sample 2 ~ 28°C - All-in-one cartridge (NA extraction + amplification) - Simultaneous detection of M. tuberculosis and drug-resistance against rifampicin (RIF) and isoniazid (INH) - Fast result in...
Open the catalog to page 10
STANDARD™ M10 Arbovirus Panel is a multiplex real-time RT-PCR test intended for use with STANDARD M10 system for the qualitative detection of Arbovirus; Dengue virus(DENV), Zika virus(ZIKV), Chikungunya virus(CHIKV), Yellow Fever virus(YFV) and West Nile virus(WNV) nucleic acids in human serum or plasma sample. Test type Specimen type Storage condition Advantage Professional Use Only Serum, Plasma 2 ~ 28°C - All-in-one cartridge (NA extraction + amplification) - Simultaneous detection of DENV, ZIKV, ChIKV, YFV and WNV - Fast result in 60 minutes - Serum / plasma sample - Room temperature...
Open the catalog to page 11
STANDARD M SARS-CoV-2/Variant I Real-Time Detection Kit is a real-time RT-PCR assay intended for the in vitro qualitative detection and differentiation of SARS-CoV-2 RNA and the Omicron variant in human nasopharyngeal swab specimens. 3D Test type Professional Use OnlySpecimen type Nasopharyngeal swabStorage condition -25 ~ -15 °CTest Performance Concentration (copies/ml) ORFlab gene N gene S gene_ins214EPE S gene_E484A Limit of Detection (LoD) - SARS-CoV-2 Wild Type ~ 1 copies/ pl - Omicron Variant ~ 2 copies/ pl Ordering InformationProducts Tests / Kit Cat. No. M SARS-CoV-2/Variant I...
Open the catalog to page 12Archived catalogs
-
SD GlucoMentor
2 Pages
-
SD GlucoNavii_EN
2 Pages
-
SD GlucoNavii NFC
6 Pages
-
2014 SD Product List
32 Pages
-
SD A1cCare
6 Pages






