
Catalog excerpts

The sound science of cartilage regeneration
Open the catalog to page 1
The advanced bioscaffold technology for enhancing cartilage regeneration A minimally invasive one-step cartilage regeneration system Suited for most cartilage lesion cases Greater quantity and better quality of tissue compared to Microfracture alone The highest standard in cartilage regeneration randomized clinical trials
Open the catalog to page 2
BST-CarGel®, naturally enhancing the process of cartilage regeneration
Open the catalog to page 3
BST-CarGel® Description ■ BST-CarGel® is an easy to use, off-the-shelf product applied during a one-step cartilage repair procedure ■ BST-CarGel® is easily prepared by combining two components — a chitosan solution and a buffer ■ BST-CarGel® is mixed with fresh autologous whole blood just before its application to a lesion surgically-prepared by Bone Marrow Stimulation
Open the catalog to page 4
BST-CarGel® Product Preparation Draw exactly 0.3 mL from the ADD vial. Steps 1-3 can be done by a non-sterile nurse while the lesion is being surgically prepared. Inject the ADD solution in a drop-wise manner into the MIX vial. Do not shake. Leave undisturbed for a minimum of 10 minutes. Once the cartilage lesion is ready, draw 5 mL of fresh autologous blo
Open the catalog to page 6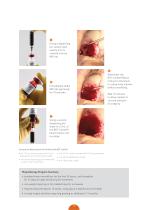
Using a dispensing pin, slowly inject exactly 4.5 mL of blood into the MIX vial. Administer the BST-CarGel®/blood mixture to the lesion in a drop-wise manner without overfilling. Immediately shake MIX vial vigorously for 10 seconds. Wait 15 minutes to allow implant to clot and maintain its integrity. Using a second dispensing pin, draw 4 to 5 mL of the BST-CarGel®/ blood mixture into a syringe. Accessories Required but not Provided with BST-CarGel® one 1.0 mL sterile syringe with 0.1 mL graduations and a 26G sterile needle two sterile dispensing pins vented with a 0.2 μm filter...
Open the catalog to page 7
Problems with Bone Marrow Stimulation Certain aspects of standard Bone Marrow Stimulation impede the cartilage repair process: blood clot retraction by as much as 50% of original volume poor residency of the blood clot in the cartilage lesion insufficient quantity of blood components necessary to drive repair BST-CarGel® Scientific Rationale Increasing the quantity and improving the residency of the blood clot in the cartilage lesion by providing a more voluminous, adherent, and physically-stabilized blood clot should improve cartilage repair outcomes after Bone Marrow Stimulation....
Open the catalog to page 8
BST-CarGel® Primary Mode of Action The mode of action of BST-CarGel® is three-fold: Acts as a scaffold to physically stabilize the blood clot in the cartilage lesion. Impedes blood clot retraction while allowing for normal clotting to occur. Adheres to the cartilage lesion surfaces. Hoemann et al., Osteoarthritis Cartilage 2007 Hoemann et al., J Bone Joint Surg Am 2005 Enhanced Secondary Healing Events Animal studies have provided extensive scientific evidence supporting the mode of action for BST-CarGel® as well as the downstream consequences of prolonging the residency of the...
Open the catalog to page 9
Clinical Sites Canada Halifax Calgary New Westminster Vancouver Winnipeg Ottawa Oakville Toronto Hamilton London Montreal Greenfield Park Quebec City St-Eustache Spain Madrid Barcelona Gijon South Korea Seoul
Open the catalog to page 10
BST-CarGel® International Clinical Trial Design Representing the first of its kind in cartilage repair, this randomized clinical trial used state-of-the-art methodologies for endpoint measurements, including novel 3-dimensional quantitative magnetic resonance imaging (MRI). Structural Co-Primary Endpoints at 12 Months quantity of repair tissue as degree of lesion filling (% Fill) by quantitative MRI quality of repair tissue by T2 mapping Secondary Endpoints at 12 Months clinical improvement by the Western Ontario and McMaster Universities Arthritis Index (WOMAC) safety by the recording...
Open the catalog to page 11
Baseline demographic characteristics of the 80 patients were similar in the two treatment groups, including age, race, gender, BMI, smoking habits, and activity levels. Compliance to the 12-week rehabilitation program was also equivalent between groups. Similar Patient Characteristics for Both Groups BST-CarGel® generally had larger lesions by quantitative MRI. Lesion volume was a prespecified statistical covariate for endpoint analysis. Lesion Volume (cm3) (cartilage+bone) Maximum Lesion Area BST-CarGel®: 6.76 cm2 Microfracture: 4.45 cm2
Open the catalog to page 12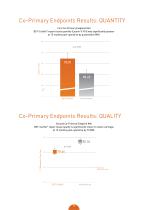
Co-Primary Endpoints Results: QUANTITY First Co-Primary Endpoint Met BST-CarGel® repair tissue quantity (Lesion % Fill) was significantly greater at 12 months post-operative by quantitative MRI. 100 Lesion % Fill Co-Primary Endpoints Results: QUALITY Second Co-Primary Endpoint Met BST-CarGel repair tissue quality is significantly closer to native cartilage at 12 months post-operative by T2 MRI. ®
Open the catalog to page 13
More Consistent Cartilage Repair with BST-CarGel® The Microfracture group had 5 times more failures (Lesion % Fill ‹ 80%) than the BST-CarGel® group. Microfracture % Fill Distribution % Fill Distribution Size Doesn’t Matter for BST-CarGel® An analysis of structural outcomes was conducted where the lesion sizes were divided into two categories, above and below 2 cm2. Quantity and quality of repair tissue after BST-CarGel® treatment are not affected by the lesion size, especially › 2 cm2. Lesion % Fill Distributions 14 Repair Tissue QUANTITY Treatment with BST-CarGel® leads to more consistent...
Open the catalog to page 14
Clinical Benefits BST-CarGel® treatment resulted in a significant clinical improvement at 12 months post-operative over baseline by WOMAC scores. 15 Baseline Post-Op Baseline Post-Op Functional Limitation Both treatment groups showed equivalent clinical improvement at 12 months post-operative. Safety The safety of BST-CarGel® was comparable to Microfracture as measured by the recording of adverse ev
Open the catalog to page 15
Biopsy Histology - Structural Parameters BST-CarGel® treatment improves repair tissue structure at 13 months by ICRS histological scoring of biopsies. Number of Improved ICRS Parameters Superior Structure Parameters BST-CarGel® vs Microfracture ICRS I, 4 of 6 ICRS II, 10 of 14 Surface Architecture Superficial Zone Basal Integration Overall Assessment Safranin-O Collagen II Collagen I Biopsy Histology - Cellular Parameters BST-CarGel® treatment improves repair tissue cellularity at 13 months by ICRS histological scoring of biopsies. Superficial Zone (SZ) Transitional Zone (TZ) Number...
Open the catalog to page 16All Smith & Nephew catalogs and technical brochures
-
ANTHEM
40 Pages
-
BIRMINGHAM HIP
32 Pages
-
FREEDOM
16 Pages
-
SALTO TALARIS
48 Pages
-
polarstem
28 Pages
Archived catalogs
-
Locking Large Fragment Overview
32 Pages
-
Locking Small Fragment Overview
68 Pages
-
Rediscover normal
4 Pages
-
TWINFIX ULTRA HA and PK
2 Pages
-
TRIGEN™ INTERTAN
12 Pages
-
EVOS SMALL Resources
12 Pages
-
NAVIO Message Brochure
8 Pages
-
Small footprint, big impact
8 Pages
-
TAYLOR SPATIAL FRAME◊
8 Pages
-
Recertification Program
8 Pages
-
anthem
4 Pages
-
Ordering information
1 Pages
-
RAPID RHINO™ NASASTENT™
6 Pages
-
clancy anatomic cruiciate
2 Pages
-
Electrosurgery
20 Pages
-
Powered Instruments
11 Pages
-
Shaver Systems
7 Pages
-
Knee
73 Pages
-
HIP
21 Pages































