 Website:
Stryker
Website:
Stryker
Group: Stryker
Catalog excerpts

Stryker Biologics Product Catalog
Open the catalog to page 1
Introduction Stryker Spine Stryker is one of the world’s leading medical technology companies and together with our customers, we are driven to make healthcare better. Stryker has collaborated with the nation’s industry-leading tissue organizations to offer a comprehensive portfolio consisting of traditional and proprietary spinal grafts and viable bone matrix, as well as demineralized bone matrix products and synthetic bone grafting substitutes. Our focus on bringing progressive, compelling products to market is guided by our quality first foundation—Stryker fulfills the rigorous...
Open the catalog to page 2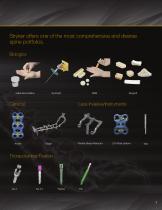
Stryker offers one of the most comprehensive and diverse spine portfolios. Biologics Viable Bone Matrix Less Invasive/Instruments Pedicle Based Retractor LITe Plate System Thoracolumbar Fixation
Open the catalog to page 3
Demineralized Bone Matrix Allograft BlO-Implants Viable Bone Matrix Bone Marrow Aspiration and Delivery Imbibe 28
Open the catalog to page 4
Viable Bone Matrix Demineralized Bone Matrix (DBM) p-TCP Maero-Mofsels. 4 (Net Volume 30c
Open the catalog to page 5
Why Choose Preservon Allograft BlO-Implants? Preservon Allograft BlO-Implants are more efficiently and conveniently stored and handled than grafts treated with conventional preservation methods. Frozen or freeze-dried allografts can require up to 60 minutes to thaw or re-hydrate, compared to as little as 30 seconds for Preservon treated Allograft BIO-Implants. The Future of Allograft BIO-Implant Preservation Preservon is a proprietary and patented glycerol-based preservation technology that allows allograft BIO-implants to be stored in a fully hydrated state at an ambient temperature. This...
Open the catalog to page 6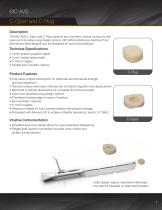
BIO AVS C-Open and C-Plug Description The BIO AVS C-Open and C-Plug implants are monolithic cortical constructs with open and cancellous plug design options. BIO AVS implants are machined from femoral and tibial allograft and are designed for cervical procedures. Technical Specifications • 12mm anterior-posterior depth • 14mm medial-lateral width • 5-10mm heights • arallel and 4˚ lordotic options P Product Features • One-piece cortical construction for optimized biomechanical strength and load dispersion • extured surface area helps minimize risk of implant migration and displacement T •...
Open the catalog to page 7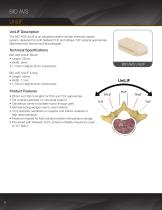
UniLIF Description The BIO AVS UniLIF is an allograft posterior lumbar interbody spacer system, designed for both bilateral PLIF and oblique TLIF surgical approaches. Machined from femoral and tibial allograft. Technical Specifications BIO AVS UniLIF (Short) • Length: 25mm • Width: 9mm • 7-15mm Heights (2mm increments) BIO AVS UniLIF (Long) • Length: 30mm • Width: 11mm • 7-15mm Heights (2mm increments) Product Features • 25mm and 30mm lengths for PLIF and TLIF approaches • Full cortical parameter for structural support • Cancellous center to facilitate fusion through graft •...
Open the catalog to page 8
BIO AVS AL Description The BIO AVS AL implant is a monolithic, cortical construct machined from femoral allograft. Designed for Anterior Lumbar Interbody Fusion (ALIF) procedures. Technical Specifications • Anterior posterior depth - 23mm minimum; 27mm maximum • Medial lateral width - 23mm minimum; 30 mm maximum • 10-18mm heights (2mm increments) • 4˚, 8˚, and 12˚ lordotic options Product Features • One-piece cortical ring for optimized biomechanical strength and load dispersion • Textured surface area helps minimize risk of implant migration and displacement • Machined to precise...
Open the catalog to page 9
Description Precision cut cancellous block with a single cortical face. Recovered from the femoral condyles, femoral heads, distal tibia, and talus. Technical Specifications • 11mm anterior-posterior depth • 14mm medial-lateral width • 5-10mm heights • Parallel Clincial Applications • Anterior Cervical Fusion Product Features • Preservon treated for fully hydrated ambient temperature storage • Provides an osteoconductive matrix for incorporation • Pre-sized for convenience and intraoperative efficiency • Processed with Allowash XG to achieve a Sterility Assurance Level to 10-6 (SAL)2...
Open the catalog to page 10
Description Tricortical graft recovered from the crest of the ilium. Technical Specifications • Minimum 25mm anterior-posterior depth • Minimum 8mm medial-lateral width • 6-10mm and 18mm heights • Parallel Clincial Applications • Anterior Cervical Fusion • Corpectomy • Ankle Fusion Product Features • Preservon treated for fully hydrated ambient temperature storage • Structural support provided by cortical aspects • Provides an osteoconductive matrix for incorporation • Pre-sized for convenience and intraoperative efficiency • Processed with Allowash XG to achieve a Sterility Assurance Level...
Open the catalog to page 11
Description BIO Shaft Femoral grafts are precision cut segments of the femoral diaphysis. Technical Specifications • 23-27mm anterior-posterior diameter • 23-30mm medial-lateral diameter • 60 and 100mm lengths Applications • Corpectomy • Fracture Management • Tumor Resection Product Features • Preservon treated for fully hydrated ambient temperature storage • Monolithic structural support provided by cortical ring • Can be customized to the specific needs of the patient • Provides an osteoconductive matrix for incorporation • Processed with Allowash XG to achieve a Sterility Assurance Level...
Open the catalog to page 12
BIOExpand is comprised of 100% demineralized cancellous bone providing an osteoconductive scaffold for cell attachment that, when rehydrated, produces a sponge-like effect allowing the graft to be compressed to fit in and around a variety of bony defects. Product features3: • Natural osteoconductive scaffold for cell attachment • Osteoinductive potential by exposed natural growth factors through the demineralization process. • Malleable, elastic, compressible when rehydrated • Ambient temperature storage • Naturally absorbs and retains bioactive fluids like blood and Bone Marrow Aspirate...
Open the catalog to page 13All Stryker catalogs and technical brochures
-
VariAx® DistalFibula
20 Pages
-
ACCOLADE® II
20 Pages
-
VariAx® 2
20 Pages
-
TruRize® Clinical Chair
2 Pages
-
TruRize™ Clinical Chair
4 Pages
-
Prime TC®
4 Pages
-
ENT navigation system
7 Pages
-
Smart Equipment Management
3 Pages
-
OrthoMap®
4 Pages
-
AxSOS 3® Titanium
36 Pages
-
Luxor
4 Pages
-
Humeral Nailing System
44 Pages
-
AVS Anchor® -C
2 Pages
-
Aviator™
2 Pages
-
Aero® -C
6 Pages
-
Escalate®
16 Pages
-
Dynatran
13 Pages
-
Reflex®
24 Pages
-
OASYS®
44 Pages
-
SurgiCount
4 Pages
-
Reusable Cuff
2 Pages
-
Disposable Cuff
2 Pages
-
SmartPump
2 Pages
-
Patient Education
2 Pages
-
Revolution
6 Pages
-
Cast Vac
2 Pages
-
Cast Cutter
2 Pages
-
Stryker NAV3i
4 Pages
-
S3 MedSurg Bed
8 Pages
-
Asnis ® Micro Xpress
2 Pages
-
EasyClip
2 Pages
-
Universal Neuro III
10 Pages
-
Right Angled Screwdriver
8 Pages
-
Neptune E-SEP
2 Pages
-
Neptune 2
2 Pages
-
InterPulse - Orthopaedics
4 Pages
-
Mixevac III
2 Pages
-
System 7 Family
7 Pages
-
SDC 3
2 Pages
-
SmartTip ™
3 Pages
-
Label Changes
2 Pages
-
Gamma3 T
6 Pages
-
the Mill
2 Pages
-
CBC II
2 Pages
-
Scorpio ®Knee TS
6 Pages
-
GMRS
13 Pages
-
trident
12 Pages
-
Gamma3 U-Blade Lag Screw
18 Pages
-
Gamma3 Fragment Control Clip
6 Pages
-
Gamma3 Long Nail R2.0
48 Pages
-
Gamma3 Trochanteric Nail 180
48 Pages
-
CD4 & SABO2 Family
5 Pages
-
System 7 Sterilization Case
2 Pages
-
System 7 Battery
2 Pages
-
System 7 Precision
2 Pages
-
System 7
7 Pages
-
ACL Instrumentation Brochure
6 Pages
-
C-Arm T racking System
2 Pages
-
company overview
12 Pages












































































