 Website:
Sugentech, Inc.
Website:
Sugentech, Inc.
Catalog excerpts

*JH\ake it Better Tomorrow A global leading in-vitro diagnostic company based on BT-IT convergence technology
Open the catalog to page 1
e it Better Tomorrow BT-IT SSPIw {dS Company Introduction INVESTOR RELATIONS 2017 I 2
Open the catalog to page 2
Sugentech, "Smart-ubiquitous gene technology" IVD products POCT technology CONVERGENCE of BT & IT | Company Status Company name | CEO Introduction ceo Mi Jin Sohn Academic background • Ph.D. at Chungnam National University School of Medicine Work experiences • Senior researcher, Technology Institute of LG Life Science • Senior fellow, Korea Research Institute of Bioscience and Biotechnology • Researcher, NIH,NIHES • Adjunct professor, Chungnam National University School of Medicine • Technical expert, Committee on medical devices, Ministry of Food and Drug Administration | Executives...
Open the catalog to page 3
2. Vision and major history A global company leading in-vitro diagnostic medical device based on a BT-IT-NT convergence technology 01 Received CE certification for digital pregnancy / ovulation tests (for the first time in Korea, TÜV SÜD) 11 Received ISO13485 certification (TÜV SÜD) 12 Established the company (Technology transfer from ETRI / Equity participation of ETRI holdings) 11 의료기기 제조업 허가as a medical Received approval device manufacturer 11 Listed in KONEX 10 Received a technology award from the Korean BioChip Society 02 Certified as a venture company 12 Won the grand prize of...
Open the catalog to page 4
3. Introduction of the currently merged company KMAC BIO Jp* • • # A ■ Established sites and facilities to expand the production capacity and now Holding the highest level of IT convergence diagnostics technologies / products | General status Mass production company A The highest level of IT convergence diagnostics technology in Korea Possessing a manufacturing site and quality system for 100 million tests production Strength of the W it company Technology A^lr Cash Co^^ Flexible sales growth
Open the catalog to page 5
4. Visions for M&A Sugentech Inc. pursues Bio-based IT convergence and KMAC BIO seeks IT-based Bio convergence Leaping forward for Global IVD company through M&A Developing the first and only digital self-testing technologies and products based on immunochemistry in Korea Developing the first and only full-automated immunochemical analysis instrument Various bio-diagnostic products Professional POCT Self-Testing Various Contents Micro-sized analysis instrument Automated large scale analysis instrument Immunochemical immunoblot instrument Professional POCT instrument Molecular diagnosis...
Open the catalog to page 6
5. Our Goal after the M&A Gloval Leading IVD Company Securing IT technology Need for advanced IT convergence technologies 〮 Balance with Bio/NT diagnostic technology 〮 Performance improvement and reducing inefficient manufacturing speed due to outsourcing Securing the new growth momentum for growth Sugentech A company specialized in POCT Securing manufacturing facilities and infrastructure Need for production capacity of 100million tests for the global market entry Need for competitiveness through high-value-added product development 〮 m-Health Dx 〮 Molecular diagnostic POCT 〮 Automation of...
Open the catalog to page 7
Bio-IT Convergence Technology Micro & low-voltage analysis system Best-in-class quantitative analysis system Korea's first digital pregnancy / ovulation tests Global Level Professional POCT Global and domestic certifications • Target design • Gene cloning • Expression, culture, purification • Ab production and screening • Application of nanoparticles • Optical array • Micro & low-voltage • Algorithm • Firmware • app Product development • The highest level of product commercialization capacity in Korea • Korea's the first digital pregnancy / ovulation tests • Best-in-class professional...
Open the catalog to page 8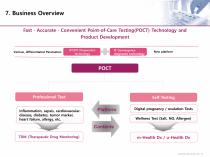
7. Business Overview Fast ∙ Accurate ∙ Convenient Point-of-Care Testing(POCT) Technology and Product Development IT Convergence diagnostic technology Various, differentiated Parameters Professional Test Inflammation, sepsis, cardiovasculardisease, diabetes, tumor marker, heart failure, allergy, etc. Digital pregnancy / ovulation Tests Wellness Test (Salt, NO, Allergen) Contents TDM (Therapeutic Drug Monitoring) INVESTOR RELATIONS 2017 9 INVESTOR RELATIONS 2017 II
Open the catalog to page 9
8. Business strategies : Market Potential Professional POCT • Full-auto product acquired its competitiveness by providing various parameters and low prices compared with the existing Lab-dedicated automation devices Price Full-Auto Semi-Auto • m-Health product secured its competitiveness by providing various services at lower prices compared to monitor types Reader Reader Visual Visual Performance Immunochemistry • It has maintained its competitiveness in niche markets for multiple diagnosis such as allergy, autoimmunity, and infectious disease Molecular Diagnosis • It is impossible to...
Open the catalog to page 10
9. Business Strategy : Key Points ODM partners for Mass market Entry • Brand marketing, CE/FDA approval • Large scale ODM for big companies (exclusive contract) • Razor-Blade business model for new products Maximization of Razor-Blade biz model Sales increase from various parameters • Introduction of new parameters such as autoimmune diagnosis, allergy, etc. • Market access with price competitiveness • New parameter development by technology collaboration Seeking of new market using molecular diagnostic Commercialization of molecular diagnostic reagent POCT • Streamlining the molecular...
Open the catalog to page 11
e it Better Tomorrow BT-IT SSPIw {dS Technology & Products INVESTOR RELATIONS 2017 I 12
Open the catalog to page 12
1. Professional POCT : Technology Unmet needs of the technology: Varieties of test parameters, Quantitative analysis, Accuracy, Usability Bio Antibody development technology Analysis algorithm development technology • Structure-specific antibody • Improvement in accuracy (patent registered) • Single molecule recognizing antibody • Neutralizing antibody • Small molecule recognizing antibody Immunoassay development technology • Immunochromatography • Enzyme-linked immunosorbent assay (ELISA) Professional POCT • Automated recognition: code chip, barcode recognition x Fluorescence / Absorbance...
Open the catalog to page 13


