 Website:
T&R Biofab
Website:
T&R Biofab
Catalog excerpts

T&R Mesh is a bio-resorbable implantable medical device. It is to assist tissue regeneration and reconstruction for the damaged or defected area. Induce tissue regeneration and reconstruction Various shapes, mechanical properties and types Provide structural support Interconnected porous structure High biocompatibility
Open the catalog to page 2
Unique StructurePowered by 3D Printing Fully interconnected pores play key roles in communication in between regenerated tissues and transportation of nutrients, allowing high efficacy and biocompatibility.
Open the catalog to page 4
Excellent Efficacy in Tissue Regeneration In-vitro studies for 21 days show the high proliferation of cells cultured on T&R Mesh. Live Cells (Green) Human osteoblasts Human septal chondrocytes Human turbinate mesenchymal stem cells
Open the catalog to page 5
PCL PCL(Polycaprolactone) is US FDA-approved(MAF-2195) biodegradable material used in a human body in a specific application. B-TCP S-TCP (beta-tricalcium phosphate) releases calcium ion to enhance the bone regeneration. Ca2+ 2 *— —*3
Open the catalog to page 6
Verified Safety T&R Mesh is KFDA approved medical device and manufactured in accordance with GMP and ISO 13485 standards. • Certified through various safety tests (extraction, cytotoxicity, genotoxicity, sterility, etc.) • Sterilized with gamma irradiation
Open the catalog to page 7
Flexible, Modifiable and Strong • T&R Mesh retains its shape and strength during surgical operations. •I Easy shaping with surgical instruments. •I No special technique is required to use T&R Mesh.
Open the catalog to page 8
General Mesh Patient Specific Implant Nasal Tip Ball Dental Membrane 10(L)*30(W)mm Customized 2~12 mm Diameter 10(L)*15(W)mm Strength • R(Regular) Customized • Single sphere type • Soft type • S(Strong) • Regular type • SS(Super Strong) • Hard type Feature Application Various sizes, thicknesses and strength. Availability of shape retailoring • CMF Reconstruction • Orbital Fracture • Nasal Augmentation Custom printed scaffold through CT image Various diameters by nasal tip size Thin film with micro pores Alveolar bone regeneration • PCL with |3-TCP • PCL with p-TCP
Open the catalog to page 9
Clinical Applications CMF (Craniomaxillofacial) Reconstruction Applicable to reconstructive treatment of orbital fracture, burr hole, craniomaxillofacial damaged area. Before Nasal Surgery Applicable to rhinoplasty and augmentation including deviated nose, septoplasty and septal extension.
Open the catalog to page 10
Oral Maxillofacial Surgery Applicable to dental GBR surgery and alveolar bone regeneration. (Patient Specific Implant) Applicable to an accurate cleft palate, ear, large facial defected area reconstruction. PSI Process Patient’s information with CT Image Modeling PSI implant Feedback 21 days Fabrication Supply a couple of PSI implants Simulation Model
Open the catalog to page 11
deCelluidTM is a Bioink made from Tissue Specific dECM(decellularized ExtraCellular Matrix) It conserves particular growth factors existing in the original tissue and promoting the regeneration of target tissue. Under development 3D printable and compatible for other bioprinters Excellent performance in cell proliferation and cytodifferentiation Thermal gelation without UV(ultra violet) irradiation
Open the catalog to page 12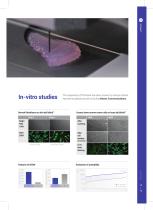
In-vitro studies The superiority of the bioink has been proven by various articles reported to global journals including Nature Communications. Dermal fibroblasts on skin deCelluid™ Day1 Human bone marrow stem cells on bone deCelluid™ After coasting After cell seeding (Morphology) F-actin Stain Live/Dead Stain Native Tissue Decell. Tissue Collagen concept(ug/mg) Live / dead staining Native Tissue Decell. Tissue 1.00
Open the catalog to page 13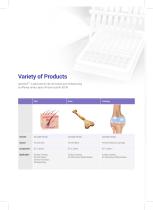
Source Porcine Skin Component Application 37'C, 30min Surface Coating 3D Cell Culture, Dermis Formation, 3D Bioprinting Sponge(100mg) Porcine Bone 37'C, 30min Surface Coating 3D Cell Culture Differentiation Sponge(100mg) Porcine meniscus cartilage 37'C, 30min Surface Coating 3D Cell Culture Differentiation
Open the catalog to page 14
In vitro primary cell cultures 3D cell culture of mammalian cells Metabolism and toxicology studies 3D bioprinting of mammalian cells The development of several types of tumor cell invasion assays The maintenance and differentiation of human embryonic stem cells and induced pluripotent stem(iPS) cells
Open the catalog to page 15
#540, SMART-HUB INDUSTRY-UNIVERSITY CONVERGECE CENTER, 237 SANGIDAEHAK-RO, SIHEUNG-SI, GYEONGGI-DO, KOREA TEL +82 31 431 3344 FAX +82 31 8041 1783 sales@tnrbiofab.com www.tnrbiofab.com
Open the catalog to page 16All T&R Biofab catalogs and technical brochures
-
TnR Nasal Mesh
8 Pages


