Website:
Telic
Website:
Telic
Group: Telic Group
Catalog excerpts

TECHNICAL DATA SHEET Product MORDEDOR-MO® Bite block, for endotracheal tubes and laryngeal masks TECHNICAL SPECIFICATIONS Materials Bite block: thermoplastic rubber bar (latex free) Pouch: PET / aluminium / PE film. Main dimensional properties Reference Sterilization and expiry date ▪ ▪ Non sterile product. Shelf life: 5 years upon the manufacturing date. Non cytotoxic (ISO 10993-5) Non irritant (ISO 10993-10) Non sensitizing (ISO 10993-10) Keep out of direct sunlight / the outside Keep in a dry place Single use product Individual pouch 50 pouches par service box Non-toxic Does not absorb moisture Latex-free Page 1 of 2 This document is the property of Telic, S.A.U. Transmission to third-parties or non-authori
Open the catalog to page 1
TECHNICAL DATA SHEET REGULATORY INFORMATION TELIC, S.A.U. guarantees that this product is in conformity with Regulation (UE)2017/745 and that it has been manufactured following the directives of the Quality Assurance System certified as ISO 13485. This product is classified as: • Class I product according to rule 5 of Annex VIII of Regulation (UE) 2017/745. • GMDN code: 10405 Seizure bite block. • EMDN code: R0199 (Intubation Devices-other). Page 2 of 2 This document is the property of Telic, S.A.U. Transmission to third-parties or non-authorized copies are not allowed. R06o-PD01
Open the catalog to page 2All Telic catalogs and technical brochures
-
CUp Electrodes
3 Pages
-
Surface electrodes
2 Pages
-
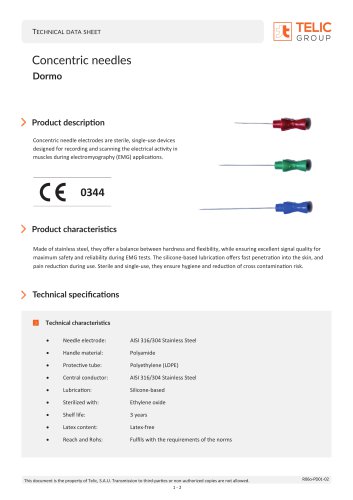
Concentric needles
2 Pages
-
Corkscrew subdermal needles
2 Pages
-
Conductive gel
3 Pages
-
Telic Groupe
27 Pages
-
Blayco dermal curette
2 Pages
-
Blayco biopsy punches
3 Pages
-
Blayco-Pad
2 Pages
-
Skin marker
2 Pages
-
Desfi-Dormo ED-1010
3 Pages
-
Dormo-Strip
3 Pages