Website:
Telic
Website:
Telic
Group: Telic Group
Catalog excerpts

TECHNICAL DATA SHEET Adhesive pre-gelled pads for defibrillation Desfi-Dormo Product description Pack of two adhesive pre-gelled pads for manual defibrillation for adult patients. Product charasteristics • This product does not contain natural latex, phthalates or compounds of animal or biologic origin. This product is in compliance with RoHS standard. Maximum permanence time recommended for contact with patient skin is 24 h. Resists up to 15 consecutive defibrillation loads at 360 J. Product intended for adult patient. Minimum weight 25 kg. Technical specifications Materials • Backing material: Waterproof flexible white PE foam ring, closed cell, with biocompatible acrylic adhesive. Conductive area: Tin foil with conductive acrylic based hydrogel, biocompatible. Packaging: PET / aluminum / PE film. Physical and dimensional properties • Active area (each pad): 140 cm2. Active area size: 134 mm x 101 mm. This document is the property of Telic, S.A.U. Transmission to third-parties or non-authorized copies are not allo
Open the catalog to page 1
TECHNICAL DATA SHEET Electrical properties • AC large signal impedance specification: <3Ω Defibrillation overload recovery: • DC offset voltage specification: <100 mV. Combined offset instability and internal noise specification: <100 μV. Bias current tolerance 8 h specification: <100 mV. Conclusion: Requirements established by electrical safety standards IEC 60601-2-4 and ANSI/AAMI DF-80 are met. Biocompatibility • ISO 10993-5: No cytotoxic. • ISO 10993-10: No sensitizing. • ISO 10993-10: Negative intracutaneous irritation test. Conclusion: The product is biocompatible. Sterilization and...
Open the catalog to page 2
TECHNICAL DATA SHEET Clean and dry application site. Avoid contact with alcohol or other disinfectants. To minimize adhesion or burn problems, shave the application area, whenever possible. Open package and remove electrodes. Remove protective film from pads. Place pads on patient at clinically indicated site, making sure that all adhesive area is stuck to the skin. Be careful not to trap any pockets of air between hydrogel and patient’s skin. Place defibrillator paddles on the metallic surface of the pads. Apply firm and uniform pressure on each of the paddles when carrying out the...
Open the catalog to page 3All Telic catalogs and technical brochures
-
CUp Electrodes
3 Pages
-
Surface electrodes
2 Pages
-
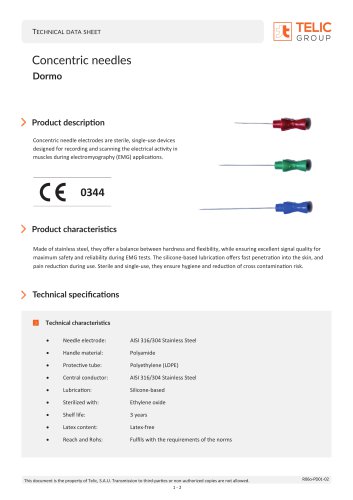
Concentric needles
2 Pages
-
Corkscrew subdermal needles
2 Pages
-
Conductive gel
3 Pages
-
Telic Groupe
27 Pages
-
Blayco dermal curette
2 Pages
-
Blayco biopsy punches
3 Pages
-
Blayco-Pad
2 Pages
-
Skin marker
2 Pages
-
Dormo-Strip
3 Pages